Abstract
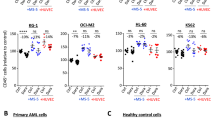
Multicenter phase II trials with Gemtuzumab Ozogamicin (GO/Mylotarg®), consisting of a CD33 antibody linked to the cytotoxic drug calicheamicin, have shown a 30% overall response rate in relapsed acute myeloid leukemia patients. However, no clear correlation was observed between CD33 expression on leukemic blasts and response to GO therapy. We analyzed the CD33 specificity of GO-induced cell death and the effect of GO on CD33-negative malignancies. We demonstrate that lysis induced by clinically relevant GO concentrations is partially CD33 mediated, and that efficient non-CD33-mediated GO uptake can occur via endocytosis. In agreement with these results, we observed GO-mediated death of human CD33-negative acute lymphoblastic leukemia cells both in vitro and in vivo in an NOD/SCID mouse model. Finally, sensitivity to GO-induced cell death was at least partially determined by the activation status of leukemic cells, with cells in activated phases of the cell cycle being most effective in both CD33-specific GO internalization, renewed expression of CD33 molecules, and non-CD33-mediated GO uptake via endocytosis. In conclusion, these data provide mechanistic insight into the efficacy of GO in CD33-positive as well as in CD33-negative malignancies with endocytic capacity, and provide a rationale for the use of GO in the treatment of malignancies with endocytic capacity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med 1995; 332: 217–223.
Lowenberg B, Downing JR, Burnett A . Acute myeloid leukemia. N Engl J Med 1999; 341: 1051–1062.
Appelbaum FR . Antibody-targeted therapy for myeloid leukemia. Semin Hematol 1999; 36: 2–8.
Sievers EL . Clinical studies of new ‘biologic’ approaches to therapy of acute myeloid leukemia with monoclonal antibodies and immunoconjugates. Curr Opin Oncol 2000; 12: 30–35.
Bernstein ID . Monoclonal antibodies to the myeloid stem cells: therapeutic implications of CMA-676, a humanized anti-CD33 antibody calicheamicin conjugate. Leukemia 2000; 14: 474–475.
Freeman SD, Kelm S, Barber EK, Crocker PR . Characterization of CD33 as a new member of the sialoadhesin family of cellular interaction molecules. Blood 1995; 85: 2005–2012.
Dinndorf PA, Andrews RG, Benjamin D, Ridgway D, Wolff L, Bernstein ID . Expression of normal myeloid-associated antigens by acute leukemia cells. Blood 1986; 67: 1048–1053.
Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF . A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leukemia Res 1984; 8: 521–534.
Wagner JE, Collins D, Fuller S, Schain LR, Berson AE, Almici C et al. Isolation of small, primitive human hematopoietic stem cells: distribution of cell surface cytokine receptors and growth in SCID-Hu mice. Blood 1995; 86: 512–523.
Andrews RG, Singer JW, Bernstein ID . Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med 1989; 169: 1721–1731.
McGavin JK, Spencer CM . Gemtuzumab ozogamicin. Drugs 2001; 61: 1317–1322.
Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody- calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjugate Chem 2002; 13: 47–58.
van der Velden V, te Marvelde JG, Hoogeveen PG, Bernstein ID, Houtsmuller AB, Berger MS et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 2001; 97: 3197–3204.
Zein N, Sinha AM, McGahren WJ, Ellestad GA . Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double- stranded DNA site specifically. Science 1988; 240: 1198–1201.
Nicolaou KC, Smith AL, Yue EW . Chemistry and biology of natural and designed enediynes. Proc Natl Acad Sci USA 1993; 90: 5881–5888.
Sievers EL, Larson RA, Stadtmauer EA, Estey E, Lowenberg B, Dombret H et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001; 19: 3244–3254.
Larson RA, Boogaerts M, Estey E, Karanes C, Stadtmauer EA, Sievers EL et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia 2002; 16: 1627–1636.
Jilani I, Estey E, Huh Y, Joe Y, Manshouri T, Yared M et al. Differences in CD33 intensity between various myeloid neoplasms. Am J Clin Pathol 2002; 118: 560–566.
Rajvanshi P, Shulman HM, Sievers EL, McDonald GB . Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood 2002; 99: 2310–2314.
Leopold LH, Berger MS, Feingold J . Acute and long-term toxicities associated with gemtuzumab ozogamicin (mylotarg(r)) therapy of acute myeloid leukemia. Clin Lymphoma 2002; 2 (Suppl 1): S29–S34.
Jedema I, Barge RMY, Nijmeijer BA, Willemze R, Falkenburg JHF . Recruitment of leukemic cells from G0 phase of the cell cycle by interferons results in conversion of resistance to daunorubicin. Leukemia 2003; 17: 2049–2051.
Nijmeijer BA, Mollevanger P, Zelderen-Bhola SL, Kluin-Nelemans HC, Willemze R, Falkenburg JH . Monitoring of engraftment and progression of acute lymphoblastic leukemia in individual NOD/SCID mice. Exp Hematol 2001; 29: 322–329.
Lange B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood 1987; 70: 192–199.
Jedema I, Barge RM, Willemze R, Falkenburg JH . High susceptibility of human leukemic cells to Fas-induced apoptosis is restricted to G(1) phase of the cell cycle and can be increased by interferon treatment. Leukemia 2003; 17: 576–584.
Duperrier K, Eljaafari A, Dezutter-Dambuyant C, Bardin C, Jacquet C, Yoneda K et al. Distinct subsets of dendritic cells resembling dermal DCs can be generated in vitro from monocytes, in the presence of different serum supplements. J Immunol Methods 2000; 238: 119–131.
Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS . Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol 2001; 41: 1206–1214.
Jordan CT, Yamasaki G, Minamoto D . High-resolution cell cycle analysis of defined phenotypic subsets within primitive human hematopoietic cell populations. Exp Hematol 1996; 24: 1347–1355.
van Oijen MG, Medema RH, Slootweg PJ, Rijksen G . Positivity of the proliferation marker Ki-67 in noncycling cells. Am J Clin Pathol 1998; 110: 24–31.
Danova M, Riccardi A, Mazzini G . Cell cycle-related proteins and flow cytometry. Haematologica 1990; 75: 252–264.
Landberg G, Tan EM, Roos G . Flow cytometric multiparameter analysis of proliferating cell nuclear antigen/cyclin and Ki-67 antigen: a new view of the cell cycle. Exp Cell Res 1990; 187: 111–118.
Schwarting R, Gerdes J, Niehus J, Jaeschke L, Stein H . Determination of the growth fraction in cell suspensions by flow cytometry using the monoclonal antibody Ki-67. J Immunol Methods 1986; 90: 65–70.
Naito K, Takeshita A, Shigeno K, Nakamura S, Fujisawa S, Shinjo K et al. Calicheamicin-conjugated humanized anti-CD33 monoclonal antibody (gemtuzumab zogamicin, CMA-676) shows cytocidal effect on CD33-positive leukemia cell lines, but is inactive on P-glycoprotein-expressing sublines. Leukemia 2000; 14: 1436–1443.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jedema, I., Barge, R., van der Velden, V. et al. Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia 18, 316–325 (2004). https://doi.org/10.1038/sj.leu.2403205
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2403205
Keywords
This article is cited by
-
Potential of antibody–drug conjugates (ADCs) for cancer therapy
Cancer Cell International (2022)
-
An update on antibody–drug conjugates in urothelial carcinoma: state of the art strategies and what comes next
Cancer Chemotherapy and Pharmacology (2022)
-
Electrostatic anti-CD33-antibody–protamine nanocarriers as platform for a targeted treatment of acute myeloid leukemia
Journal of Hematology & Oncology (2022)
-
Unlocking the potential of antibody–drug conjugates for cancer therapy
Nature Reviews Clinical Oncology (2021)
-
Navitoclax enhances the effectiveness of EGFR-targeted antibody-drug conjugates in PDX models of EGFR-expressing triple-negative breast cancer
Breast Cancer Research (2020)