Abstract
The efficacy and safety of a selective NK1 antagonist, L-759274, was investigated in outpatients with diagnosis of major depressive disorder with melancholic features, following evidence obtained with the novel compound aprepitant that Substance P (NK1) antagonists may provide a unique mechanism of antidepressant activity. A randomized, double-blind placebo-controlled study was carried out. Patients, male or female, aged 18–60, scoring ⩾25 points on total of first 17 items of 21-item Hamilton Depression Scale (HAMD), and scoring ⩾4 (moderately ill) on Clinical Global Impressions-Severity Scale were randomized to oral L-759274 40 mg daily (n=66) or placebo (n=62) for 6 weeks. For patients receiving L-759274, improvement (mean decrease from baseline) in HAMD-17 total score was 10.7 points, compared with a mean 7.8 point improvement in patients receiving placebo (p<0.009). Mean scores for item 1 of HAMD-17 (depressed mood) also improved to a greater extent in the active group compared with the placebo group (0.3 points, p<0.058). Compared with placebo, mean scores on Clinical Global Impressions-Improvement Scale improved significantly by the end of the trial (p=0.009). L-759274 was generally safe and well-tolerated. The incidence of sexual side effects was on par with that observed in patients receiving placebo, and the incidences of gastrointestinal effects were low. Antidepressant actions have now been observed with two different highly selective NK1 antagonists (aprepitant and L-759274). NK1 antagonism is a replicated and generally well-tolerated antidepressant mechanism.
Similar content being viewed by others
INTRODUCTION
Biogenic amine hypotheses of depression have prevailed since Schildkraut (1965) and Bunney and Davis (1965) published the ‘catecholamine hypothesis’ in 1965. Subsequent literature (eg Arora and Meltzer, 1989; Deakin et al, 1990; Delgado et al, 1989; Maes et al, 1990; Meltzer and Lowy, 1987; Møller et al., 1990; Sarrias et al, 1987; Upadhyaya et al, 1991; Young et al, 1985) suggested that serotonergic mechanisms may in part underlie vulnerability to major depression (and some anxiety disorders), and that agents that increase serotonergic activity may be therapeutic. Functional underactivity in dopamine transmission has also been comparatively recently suggested (Randrup et al, 1975; Willner, 1983) to contribute to the pathology of depression. Although the number of neurochemical systems implicated in the pathophysiology of major depression has multiplied over the years, these principally involve classic biogenic monoamine systems. Despite innovative hypotheses that attempt to unify current knowledge, (Duman et al, 1997; Owens, 1996–1997; Richelson, 1996; Risch, 1997), a clear explanation of the pathophysiology of depression remains elusive.
A growing body of evidence now suggests that Substance P (SP), an undecapeptide neuropeptide of the neurokinin family, may play a role in depression and anxiety. It has been shown that limbic binding sites for SP are colocalized with those of monoamine neurotransmitters (Dietl and Palacios, 1991; Elliot et al, 1986; Gerard et al, 1991; Hershey and Krause, 1990; Hökfelt, 1991; Hopkins et al, 1991; Masu et al, 1987; Takeda and Krause, 1991; Yokota et al, 1989). SP displays highest affinity for tachykinin, type 1 [NK1] receptors (Otsuka and Yoshioka, 1993). It has long been considered to be a specialized sensory transmitter, which is released when an organism is exposed to noxious stimuli (Lim, 1966; Maggi, 1995) and that seems to synchronize physiologic ‘survival-type’ (stress) responses such as might occur upon noxious stimulation (Boyce et al, 2001; Culman and Unger, 1995; Shaikh et al, 1993; Smith et al, 1999; Unger et al, 1988.) We thus speculated that in the absence of actual trauma or noxious stimuli, excessive SP activity in key limbic circuits might spawn a cascade of psychophysiological activity, producing signs and symptoms such as those observed in anxiety or depression. Accordingly, antagonism of dysregulated SP activity at the NK1 receptor, the only neurokinin receptor known to be clearly expressed in human brain to date, might provide a novel mechanism for antidepressant and/or anxiolytic effects. Preclinical behavioral pharmacology studies with tachykinin NK1 receptor antagonists (Cheeta et al, 2001; Cutler, 1994; Regoli et al, 1994; Roccon et al, 1995; Vassout et al, 1994) have been complicated by species variants in receptor pharmacology. However, behavioral studies (Kramer et al, 1998) utilizing brain-penetrant NK1 antagonists in a species (guinea pig) with NK1 pharmacology similar to that seen in humans provided evidence that selective blockade of the NK1 receptor was associated with an antidepressant-like profile. This gave support for our initial speculations and for the subsequent evaluation of NK1 receptor antagonists in patients diagnosed with major depression. Aprepitant (also known as MK-0869) was chosen to test the concept initially in patients because it is orally available, well tolerated, and shows high affinity and selectivity as an antagonist at human NK1 receptors (Cascieri et al, 1992, 1985; Sadowski et al, 1993; Tattersall et al, 1993, 1994. Importantly, aprepitant and its metabolites were shown not to inhibit monoamine metabolism, and to be without direct activity at previously established molecular targets for antidepressants such as the monoamine transporter systems. In a 6-week double-blind, placebo-controlled trial, we demonstrated that aprepitant effectively treated outpatients with major depression and was generally safe and very well-tolerated (Kramer et al, 1998).
Herein, we report a multicenter study in which the efficacy, safety, and tolerability of another highly selective NK1 antagonist, L-759274, was examined to replicate the concept of NK1 antagonism as an antidepressant mechanism. Patients treated in the current study had significant major depression that included melancholic features, and which would be considered to be a biologically based (endogenous) and difficult-to-treat form of depression. It was therefore anticipated that the study would provide a rigorous test of the antidepressant efficacy of L-759274. In addition, because the placebo response in this population is generally low (Ghosh and Kramer, 1999; Peselow et al, 1992; Tignol et al, 1992) and because the diagnosis is generally unambiguous, we expected that the study results would be definitive.
L-759274 is a high-affinity (low nM range), selective, orally bioavailable nonpeptide SP (NK1) antagonists (SPAs) with a well-tolerated side effect profile in studies in normal volunteers. Like aprepitant, L-759274 lacks direct affinity for noradrenergic or serotonergic receptors, reuptake transporters, monoamine oxidase, or other enzymes that catabolize monoamines. Aside from structural differences, L-759274 and aprepitant are similar pharmacologically in terms of their high selectivity for NK1 receptors.
MATERIALS AND METHODS
Informed Consent, Subjects, and Enrollment Criteria
Prior to initiation, Investigational Review Boards approved the study protocol at each investigative site. Investigators discussed the requirements and restrictions of the study with the patients and obtained oral and written informed consent. Male and female depressed outpatients between the ages of 18 and 60 in good physical health were eligible for the study. Female patients were required to use adequate contraceptive methods during the study. Patients considered to be at risk for suicide or violence were not enrolled in the study. Patients were carefully monitored during the study and were afforded the option of continuing medical care after their participation in the study was concluded.
Diagnostic Assessment and Severity of Illness at Baseline
Patients were required to have had a DSM-IV diagnosis of major depressive disorder (MDD) (single or recurrent) (American Psychiatric Association, 1994) with melancholic features as determined by a standard clinical interview, a current episode of ⩾4 weeks, and the following scores at screening and end-of-washout visits: ⩾25 on the first 17 items of the Hamilton Rating Scale for Depression (HAMD, a standard validated instrument for quantifying depression), and ⩾4 (moderately ill) on the Clinical Global Impressions-severity of illness (CGI-S) scale (used to grade the total clinical response) (Early Clinical Drug Evaluation Unit [ECDEU] Assessment Manual, 1976).
General Study Design
This 6-week, randomized, double-blind, placebo-controlled trial in outpatients with MDD was conducted at nine investigative sites. All psychiatric investigators were experienced in conducting clinical trials in depressive disorders. Patients completed a 7-day (±3 days) washout of previous psychotropic medications (except for 4 weeks for fluoxetine; 2 weeks for monoamine oxidase inhibitors). At the screening visit, safety and efficacy assessments were obtained and compared with inclusion/exclusion criteria. At the end of the washout period, patients were re-examined for continuing eligibility (ie the HAMD and other key efficacy and safety evaluations were administered at the initial screening visit and at the end of washout); qualified patients were randomized in equal numbers to receive either oral L-759274 40 mg or matching oral placebo administered once daily in the evening for 6 weeks.
Medications and Plasma Levels
All medications were to be taken orally, once daily in the evening, for 6 weeks. L-759274 40 mg tablets with matching placebo were used. All other antidepressant or mood-stabilizing medications were prohibited during the course of the study. Based on human positron emission tomographic imaging of NK1 receptors, 40 mg of the NK1 antagonist was predicted to achieve high occupancy of central NK1 receptors.
Assessments
Patients were required to visit the clinic for a screening session, a baseline visit a week later, and weekly thereafter; efficacy measurements were made at the end of weeks 1, 2, 4, and 6 or termination. The primary efficacy outcome measure of this study was the total score on the 17-item version of the HAMD; secondary measures included the Hamilton Rating Scale for Anxiety (HAM-A) total score and the CGI-I (previously described.) In addition, an alternate standard efficacy rating scale for depression, the Montgomery Asberg Depression Rating Scale (MADRS) was also used. Efficacy assessments were performed by a psychiatrist or an experienced psychometric rater. Safety and tolerability measures included physical examination, weight, laboratory evaluations, vital signs, ECG at specified intervals, and adverse experience reporting. Information about adverse experiences was obtained by questioning the patient at each clinic visit. Any adverse experiences were rated by the study staff, in a blinded fashion, with regard to seriousness (regulatory definition), severity, duration, and drug-relatedness based on the patient's reports.
Statistical Analyses
The predefined primary efficacy analysis compared the mean changes from baseline between L-759274 and placebo on total score of the HAMD-17 at Week 6 based on an all-patients-treated last-observation-carried-forward (LOCF) approach. Based on a two-tailed test with α=0.05, the power to detect a four-point difference of change in total HAMD-17 between L-759274 and placebo was 80%, based on a standard deviation of 8.0 for the HAMD-17 changes from baseline. Pairwise comparison p-values (L-759274 vs placebo) were calculated at each week using an analysis of variance method. The analysis model included treatment group, investigative site, and randomization stratum (baseline CGI-S=4 or ⩾5) as factors. Pairwise statistical comparisons were performed using a two-tailed significance level of 0.05. No multiplicity adjustment was performed, since there was one primary hypothesis of efficacy.
Similar analyses were used to evaluate secondary efficacy variables including HAM-A, CGI-I, and each item and factor of the HAMD-17. A supportive analysis was conducted using a longitudinal data analysis method. This method, which included all intermediate data between baseline and Week 6, provided estimates of treatment effect expected at Week 6 if all patients had completed the study as planned. The model included factors of investigative site, treatment, week as a categorical variable, and week-by-treatment and week-by-center interactions. An unstructured covariance matrix was used for the within-subject correlation. The treatment differences with respect to change from baseline were estimated and tested based on a maximum likelihood approach. This model assumed that any missing data were missing at random (ie the absence of a data point was not related to the missing data itself; this assumption was presumed to be valid for data missing due to premature discontinuation of patients from the study).
RESULTS
Baseline Comparability: Patient Characteristics
Baseline characteristics for all patients randomized are shown in Table 1. The mean age of patients in this study was ∼40 years (SD, 10). Although patients in the L-759274 group were slightly younger on average (mean age: 38 years) than the patients in the placebo group (mean age: 42 years), the difference was not statistically significant. In all, 68% of the patients in the study, approximately 74% of patients in the L-759274 group and 61% in the placebo group, were female (p=NS). The majority of patients (70%) were Caucasian. Baseline HAMD and HAM-A scores were similar in each treatment group.
Patient Accounting/Reasons for Discontinuing
A total of 237 patients were screened, and of these 128 were randomized into the study. The remaining patients either did not meet study inclusion/exclusion criteria or decided not to participate. Of the 128 patients randomized, 117 had at least one psychometric evaluation of the HAMD-17 after randomization and receiving at least one treatment, and were therefore included in the primary efficacy analyses. Table 2 displays the number of patients completing the study and the number of patients discontinuing the study, along with the reasons for discontinuing. Overall, about 48 patients (38%) discontinued the study prematurely, in approximately comparable numbers in each treatment group. There were no statistically significant differences between groups for specific reasons for discontinuation.
Efficacy Results
Total HAMD-17 scores
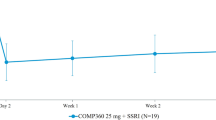
The mean changes from baseline (standard errors of the mean, SE) for the total score of the HAMD-17, the predefined primary outcome measure for this study, are displayed by week in Figure 1 for each treatment condition. The mean baseline HAMD total scores of ∼28 points in both groups represent a significant degree of depressive illness. An antidepressant effect was evident after 1 week of treatment with L-759274 (p=0.031 vs placebo), and the maximum effect within the confines of the time period studied was observed between 4 and 6 weeks after treatment. This maximum effect based on the LOCF approach and the ANOVA model including terms for treatment, investigative site, and stratum was an average improvement of 3.4 points compared to placebo (95% CI: [−5.9, −0.9], p=0.009.) Importantly, the efficacy of L-759274 over placebo in terms of total HAMD score was evident at seven out of nine of the independent investigative sites. When considering all observations (ie Weeks 1–6) in the longitudinal data analysis, the treatment effect estimate was −4.1 points (p=0.006). The above results are also supported using the data-as-observed approach (−3.5 point difference in improvement between groups, 95% CI: [−6.7, −0.2], p=0.035). This similarity between the LOCF and data-as-observed and longitudinal analyses is consistent with the finding that rates of discontinuation for various reasons were similar at each timepoint, indicating that the efficacy of L-759274 as assessed by the LOCF technique was not due to differential dropout rates between the groups.
Mean changes based on the total of all 21 items of the HAMD scale were similar to those obtained on the total of the first 17 items (−4.0 points, SE=1.4, 95% CI: [−6.7, −1.3], p=0.004).
The percent change from baseline HAMD-17 score is shown in Figure 2 for each treatment group as part of the cumulative percent change from baseline to Week 6. The figure suggests that the greatest difference between groups occurred from about −30 to −50% improvement from baseline. For example, the percentage of patients with at least 50% improvement from baseline (in HAMD-17 score) receiving L-759274 was 37 vs 25% taking placebo (odds ratio 2.2; p=0.076 vs placebo); 44% of patients in the group receiving L-759274 had at least a 40% improvement vs 24% of patients in the placebo group.
Cumulative distribution of percent change from baseline for Week 6 HAMD-17. Dashed line=placebo, solid line=L-759274 40 mg. The figure displays the cumulative percentage of patients achieving a certain level of response. For example, a common number chosen to categorize a response is 50%. If a vertical line is drawn from the −50 mark (x-axis) to the curves, the percentage of patients who achieved 50% response can be determined by drawing horizontal lines over to the y-axis. If one is interested in determining the percentage of patients with a different level of response (eg 20%), this can also be constructed from the graph using a similar procedure.
HAMD factors
Mean changes from baseline to Week 6 for the four factors of the HAMD-21 are shown in Table 3. In general, mean improvements on all factors were numerically superior in patients receiving L-759274 compared with placebo, and in this sample were significantly superior to placebo in terms of subjective aspects of depressed and anxious mood, as well as somatic aspects of depression.
HAMD individual items
Mean changes from baseline to Week 6 for each of the 21 individual items of the HAMD-21 were explored in order to characterize the profile of L-759274 further compared with placebo. These secondary findings should be interpreted cautiously, given the multiplicity of measures examined (no adjustment for multiplicity was made); therefore, p-values presented in these analyses should be viewed as exploratory, rather than as conclusive evidence of effect. Patients receiving L-759274 showed numerically superior improvement compared with those taking placebo on depressed mood (p=0.058), suicidal ideation (p=0.008), initial insomnia, (p=0.028), agitation (p=0.064), retardation (p=0.060), anxiety/psychic (p=0.002), hypochondriases (p=0.014), diurnal variation severity (p=0.036), and obsessive–compulsive symptoms (p=0.012). There was no item for which placebo treatment was superior to L-759274. Thus, the spectrum of antidepressant activity of L-759274 appeared to be quite broad and included improvements in cognitive, emotional, and physical/biological aspects of major depression.
CGI-I
By Week 6 of the study, patients receiving L-759274 40 mg were judged on average by investigators to have shown improvement (average of 2.7 points), compared with patients receiving placebo (p=0.009), who were considered on average to have achieved no change to minimal improvement (3.2 points).
In terms of CGI-I responders, a total of 47% (29 out of 62) of patients receiving L-759274 achieved a CGI-I score of 1 (very much improved) or 2 (much improved) at Week 6, compared with 21% (12 out of 56) of patients taking placebo (odds ratio 3.2, p=0.014)
MADRS
The high baseline MADRS scores for the L-759274 and placebo groups (33.4 and 34.7 points, respectively) are consistent with the high HAMD baseline values. By the end of the trial, the mean total MADRS score was 22.2 points in patients receiving L-759274 and 27.0 points in patients receiving placebo. The difference in change from baseline scores of 3.7 points (−11.3 points for L-759274 vs −7.7 points for placebo) was statistically significant (p=0.042).
HAM-A
Mean changes from baseline and SE on the HAM-A for patients receiving L-759274 40 mg or placebo are depicted in Figure 3. The anxiolytic effect of L-759274 is numerically evident as early as Week 1. At Week 6, the estimated difference between L-759274 40 mg and placebo is statistically significant (−2.8 points, p=0.027).
These results are also consistent with the improvements on the Anxiety/Somatic factor of the HAMD and item #10 of the HAMD (Anxiety/Psychic), as shown in Table 3, in patients who received L-759274 compared with those who received placebo.
Safety/Tolerability
In this study, L-759274 was generally safe and well-tolerated. The numbers (%) of patients reporting one or more adverse experiences were 50 (76%) and 40 (65%) in the L-759274 and placebo groups, respectively. Only six patients, four in the L-759274 group and two in the placebo group, discontinued the study on the basis of clinical adverse experiences. No single adverse experience caused discontinuation in more than one patient receiving L-759274 and no serious adverse experiences were reported for patients receiving L-759274.
The three most commonly (ie incidence ⩾10%) reported adverse experiences for L-759274 and placebo were headache (17 and 15%, respectively, p=NS), somnolence (17 and 2%, respectively, χ2=11.4, p<0.05), and nausea (14 and 7%, respectively, p=NS).
There were no reports of drug-seeking behavior, symptoms of drug withdrawal, or any other clinical adverse experiences suggestive of drug abuse. No pattern of clinically significant changes in vital signs, physical examination, weight, or electrocardiograms in patients treated with L-759274 was observed.
No systematic pattern of laboratory abnormalities was observed for patients receiving L-759274 or placebo. No specific type of laboratory adverse experience occurred in more than one patient.
DISCUSSION
These data suggest that L-759274, a highly selective nonpeptide NK1 antagonist, is an efficacious and well-tolerated antidepressant. The present study provided a stringent test of the antidepressant efficacy, as the patients who benefited from L-759274 suffered from a medically significant form of depression, that is, severe MDD with melancholic features, which is often difficult to treat. Importantly, the results of this study confirm previous findings with aprepitant (Kramer et al, 1998): that antagonism of the NK1 receptor in depressed patients leads to antidepressant effects and anxiolytic effects.
The principal result of this study, a 3.4-point difference in mean change from baseline to Week 6 between L-759274 and placebo on the total HAMD, satisfied the primary hypothesis for this study. Notably, the antidepressant effect of L-759274 was evident as early as Week 1 (p=0.03), and it increased to a statistically and clinically relevant difference from placebo by Week 6 (p=0.009). The antidepressant effect of L-759274 as measured by the total score of the HAMD-17 was corroborated by similar trends on all secondary measures including HAMD items (eg item #1, depression), HAMD factors, CGI-I, and MADRS. As hypothesized, L-759274 also demonstrated anxiolytic activity in this patient population. By Week 6, this anxiolytic effect was statistically significant.
The overall tolerability of L-759274 was generally similar to that observed in patients treated with placebo. A numerical improvement in sleep disturbance (statistically significant for item #4 of the HAMD, early insomnia) was observed in patients receiving L-759274 compared with those receiving placebo. Notably, neither somnolence nor cognitive impairment (as assessed by standard screening psychomotor tests), was observed in normal volunteers dosed with L-759274 40 mg in the evening (unpublished data, Merck Research Laboratories).
When sleep-related items of the HAMD (#4, 5, and 6) were deleted from the total score in these studies, overall antidepressant efficacy was still apparent, thus indicating that the efficacy of L-759274 or aprepitant is of broader consequence than is represented by their therapeutic effects on sleep alone.
No sexual dysfunction was observed in patients receiving L-759274. This is clinically significant as sexual dysfunction is a major problem associated with the use of many commonly prescribed antidepressants (Montejo-Gonzalez et al, 1997). The incidence of nausea, which was mild and transient, occurred more frequently (14%) in patients receiving L-759274 than in patients receiving placebo (7%). To put this finding in perspective, the incidence of nausea is generally ∼30% or more with serotonergic antidepressants and is the leading cause of short-term discontinuation of treatment with SSRIs (Trindade et al, 1998). However, no patient receiving L-759274 discontinued due to gastrointestinal side effects. Thus, the tolerability profile of L-759274 is generally consistent with what had been observed with aprepitant: gastrointestinal side effects (mainly nausea) and sexual dysfunction occurred less frequently than with paroxetine (20 mg/day), a benchmark of the SSRI class. It should be cautioned, however, that the present study was relatively small and was not powered to detect differences between groups with regard to adverse experiences.
There are several caveats to the interpretation of these data. Firstly, the study lacked a positive antidepressant control. Nevertheless, the observed quantified outcomes on various psychometric measures of efficacy in this study are consistent with the activity of an antidepressant, not a nonspecific agent (Lemoine et al, 1991). In addition, the time course of response, although observed early in the course of treatment, was gradually progressive as typically seen with antidepressants. In this study, the ratio of responsive patients (as defined by a 50% improvement in HAMD-17 score from baseline), receiving L-759274 compared with those receiving placebo was 1.5 (ie 37 vs 25%), comparable to that reported in the only other published placebo-controlled study in outpatients with severe depression, melancholic subtype (Heiligenstein et al, 1994). These results are also generally comparable with those obtained in two placebo-controlled studies of known active antidepressants in inpatients with major depression with melancholic features (Peselow et al, 1992; Tignol et al, 1992).
Secondly, we considered the possibility that efficacy ratings might have been confounded by the side effects of L-759274, although those effects were generally mild. However, placebo-corrected changes from baseline in HAMD-17 were strictly comparable in patients who were judged by investigators to have had drug-related adverse experiences compared with those who did not (∼−3 HAMD-17 placebo-corrected points, each). Interestingly, patients with somnolence (N=12) (ratio L-759274 to placebo, 11 : 1), experienced an overall worsening (∼+1.5 points) compared with those patients who reported no somnolence in the study (N∼105) and with patients who achieved an overall improvement in placebo-corrected HAMD-17 of about −5 points.
There are several plausible mechanisms by which SPAs exert antidepressant effects. First, SP has direct actions in brain regions that orchestrate stress responses, such as the amygdala and hypothalamus (Boyce et al, 2001; Culman and Unger, 1995; Shaikh et al, 1993; Smith et al, 1999; Unger et al, 1988). It is likely that known antidepressants act through a final common pathway that includes the action of SP at the NK1 receptor. It has been previously shown that antidepressants of various classes reduce levels of SP in several brain regions, including limbic structures (Barden et al, 1983; Brodin et al, 1987; Shirayama et al, 1996). Although the mechanism for this reduction is presently unclear, classical antidepressants do not block the NK1 receptor (Kramer et al, 1998). Secondly, preclinical electrophysiological studies show that SPAs regulate serotonergic (Conley et al, 2002; Froger et al, 2001; Santarelli et al, 2001) and noradrenergic (Hahn and Bannon, 1999; Maubach et al, 2002) neuronal activity, but in a manner that is distinct from that seen with established monoamine reuptake inhibitors. Thirdly, data suggest that SPAs may produce additive or synergistic effects with established antidepressant drugs. Data in favor of this possibility include two observations: (1) fluoxetine and a centrally acting NK1 antagonist synergistically inhibit stress-induced vocalization in guinea-pig pups (unpublished data, Merck Research Laboratories); and (2) the ability of fluoxetine to increase extracellular 5-HT efflux is considerably greater in NK1R−/− than wild-type mice (Froger et al, 2001).
The results of the clinical study reported herein, complemented by recent preclinical mechanistic studies briefly summarized above, indicate that NK1 antagonism (SPAs) may serve as a primary mechanism for the treatment of patients with depression, and that the SPA mechanism is generally very well-tolerated clinically. Further studies will be required to elucidate the possible advantages of this class fully in terms of a favorable side-effect profile, rapidity of action on key features of depression, broad efficacy across the severity spectrum of depression, anxiolytic effects in depression, enhanced long-term compliance, reduced relapse rates in long-term treatment, and efficacy when combined with standard antidepressants.
References
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders 4th edn American Psychiatric Association: Washington, DC.
Arora RC, Meltzer HY (1989). Increased serotonin 2(5-HT2) receptor binding as measured by 3H-lysergic acid diethylamide (3H-LSD) in the blood platelets of depressed patients. Life Sci 44: 725–734.
Barden N, Daigle M, Picard V, Di Paolo T (1983). Perturbation of rat serotonergic systems results in an inverse relation between substance P and serotonin concentrations measured in discrete nuclei. J Neurochem 41: 834–840.
Boyce S, Smith D, Carlson E, Hewson L, Rigby M, O'Donnell R et al (2001). Intra-amygdala injection of the substance P (NK1 receptor) antagonist L-760735 inhibits neonatal vocalization in guinea-pigs. Neuropharmacology 41: 130–137.
Brodin E, Ogren SO, Theodorsson-Norheim E (1987). Effects of subchronic treatment with imipramine, zimelidine, and alaprocalate on regional tissue levels of substance P – and neurokinin A/neurokinin B-like immunoreactivity in the brain and spinal cord of the rat. Neuropharmacology 26: 581–590.
Bunney Jr WE, Davis JM (1965). Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry 13: 483–494.
Cascieri MA, Bier E, Fong TM, Sadowski S, Bansal A, Swain C et al (1992). Characterization of the binding of a potent, selective, radioiodinated antagonist to the human NK1 receptor. Mol Pharmacol 42: 458–463.
Cascieri MA, Chichi GG, Liang T (1985). Demonstration of two distinct tachykinin receptors in rat brain cortex. J Biol Chem 260: 1501–1507.
Cheeta S, Tucci S, Sandhu J, Williams AR, Rupniak NMJ, File SE (2001). Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils. Brain Res 915: 170–175.
Conley R, Cumberbatch MJ, Mason GS, Williamson DJ, Harrison T, Locker A et al (2002). Substance P (NK1) receptor antagonists enhance dorsal raphe neuronal activity. J Neuro Sci 22: 7730–7736.
Culman J, Unger T (1995). Central tachykinins: mediators of defense reaction and stress reactions. Can J Physiol Pharmacol 73: 885–891.
Cutler MG (1994). Potential anxiolytic activity in gerbils from the substance P receptor antagonist, CGP 4982. J Psychopharmacol 8: Conf. Abstract: A22.
Deakin JF, Pennell I, Upadhyaya AJ, Lofthouse R (1990). A neuroendocrine study of unction in depression: evidence for biological mechanisms of endogenous and psychosocial causation. Psychopharmacology 101: 85–92.
Delgado PL, Charney DS, Price LH, Landis H, Heninger GR (1989). Neuroendocrine and behavioral effects of dietary tryptophan restriction in healthy subjects. Life Sci 45: 2323–2332.
Dietl MM, Palacios JM (1991). Phylogeny of tachykinin receptor localization in the vertebrate central nervous system: apparent absence of NK-2 and NK-3 binding sites in the human brain. Brain Res 539: 211–222.
Duman RS, Heninger GR, Nestler EJ (1997). A molecular and cellular theory of depression. Arch Gen Psychiatry 54: 597–606.
Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual (1976). Dept of Health, Education and Welfare Publication (ADM) 76–338: 583–585.
Elliot PJ, Nemeroff CB, Kilts CD (1986). Evidence for a tonic facilitatory influence of substance P on dopamine release in the nucleus accumbens. Brain Res 385: 379–382.
Froger N, Gardier AM, Moratalla R, Alberti I, Lena I, Boni C et al (2001). 5-hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J Neurosci 21: 8188–8197.
Gerard NP, Garraway LA, Eddy RL, Shows TB, Lijima H, Pacquet JL et al (1991). Human substance P receptor (NK-1): organization of the gene chromosome localization and functional expression of cDNA clones. Biochemistry 30: 10640–10646.
Ghosh K, Kramer MS (1999). A review of placebo-controlled efficacy trials for antidepressants as evaluated by the FDA. The 39th Annual Meeting of NCDEU: New Clinical Drug Evaluation Unit Program; 1999 Jun 01; Boca Raton, FL, 1999, p. 1.
Hahn MK, Bannon MJ (1999). Stress-induced C-fos expression in the rat locus coeruleus is dependent on neurokinin 1 receptor activation. Neuroscience 94: 1183–1188.
Heiligenstein JH, Tollefson GD, Faries DE (1994). Response patterns of depressed outpatients with and without melancholia: a double-blind, placebo-controlled trial of fluoxetine versus placebo. J Affect Disord 30: 163–173.
Hershey AD, Krause JE (1990). Molecular characterization of a functional cDNA encoding the rat substance P receptor. Science 247: 958–962.
Hökfelt T (1991). Neuropeptides in perspective: the last ten years. Neuron 7: 867–879.
Hopkins B, Powell SJ, Danks P, Briggs I, Graham A (1991). Isolation and characterization of the human lung NK-1 receptor cDNA. Biochem Biophys Res Commun 180: 1110–1117.
Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ et al (1998). Distinct mechanism for antidepressant activity by blockage of central Substance P receptors. Science 281: 1640–1645.
Lemoine P, Boulenger JP, Caillard V, Tanne N, Bonnet D (1991). Compared efficacy of prazepam and clomipramine in major depression with anxiety: a multicenter controlled study. Pharmacopsychiatry 24: 175–179.
Lim RKS (1966). A revised concept of the mechanism of analgesia and pain. Knighton RS Dumke PK (eds) In Pain. Little, Brown, and Co.: Boston, MA, 117–154.
Maes M, Jacobs MP, Suy E, Minner B, Leclercq C, Christiaens F et al (1990). Suppressant effects of dexamethasone on the availability of plasma L-tryptophan and tyrosine in healthy controls and in depressed patients. Acta Psychiatr Scand 81: 19–23.
Maggi CA (1995). The mammalian tachykinin receptor. Gen Pharmacol 26: 911–944.
Masu Y, Nakayama K, Tamaki H, Harada Y, Kuno M, Nakanishi S (1987). cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature 329: 836–838.
Maubach KA, Martin K, Chicchi G, Harrison T, Wheeldon A, Swain CJ et al (2002). Chronic substance P (NK1) receptor antagonist and conventional antidepressant treatment increases burst firing of monoamine neurones in the locus coeruleus. Neuroscience 109: 609–617.
Meltzer HY, Lowy MT (1987). The serotonin hypothesis of depression. In: Meltzer HY (ed) Psychopharmacology: The Third Generation of Progress. Raven Press: New York. pp 513–526.
Møller SE, Bech P, Bjerrum H, Bøjholm S, Butler B, Folker H et al (1990). Plasma ratio tryptophan/neutral amino acids in relation to clinical response to paroxetine and clomipramine in patients with major depression. J Affect Disord 18: 59–66.
Montejo-Gonzalez AL, Llorca G, Izquierdo JA, Ledesma A, Bousono M, Calcedo A et al (1997). SSRI-induced sexual dysfunction: fluoxetine, paroxetine, sertraline, and fluvoxamine in a prospective, multicenter, and descriptive clinical study of 344 patients. J Sex Marital Ther 23: 176–194.
Otsuka M, Yoshioka K (1993). Neurotransmitter functions of mammalian tachykinins. Physiol Rev 73: 229–308.
Owens MJ (1996-97). Molecular and cellular mechanisms of antidepressant drugs. Depress Anxiety 4: 153–159.
Peselow ED, Sanfilipo MP, Difiglia C, Fieve RR (1992). Melancholic/endogenous depression and response to somatic treatment and placebo. Am J Psychiatry 149: 1324–1332.
Randrup A, Munkvad I, Fog R (1975). Mania, depression and brain dopamine. In: Essman WB, Valzelli L (eds) Current Developments in Psychopharmacology Vol. 2 Spectrum Press: New York. pp 206–248.
Regoli D, Nguyen QT, Jukic D (1994). Neurokinin receptor subtypes characterized by biological assays. Life Sci 54: 2035–2047.
Richelson E (1996). Synaptic effects of antidepressants. J Clin Psychopharmacol 16(Suppl 2): IS–7S.
Risch SC (1997). Recent advance in depression research: from stress to molecular biology and brain imaging. J Clin Psychiatry 58(Suppl 5): 3–6.
Roccon A, Marchionni D, Nisato D (1995). Effects of SR 142801, the first non-peptidic NK3 receptor antagonist on cardiovascular and behavioral responses to senktide in guinea-pigs. In Pharmacological Research. Academic Press: New York. Abstracts presented at First European Congress of Pharmacology, 16–19 June 1995, Milan, Italy, p. 191.
Sadowski S, Huang R-RC, Fong TM, Marko O, Cascieri MA (1993). Characterization of the binding of [125I-iodo-histidyl methyl-phe7] neurokinin B to the neurokinin-3 receptor. Neuropeptides 24: 317–319.
Santarelli L, Gobbi G, Debs PC, Sibille EL, Blier P, Hen R et al (2001). Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci USA 98: 1912–1917.
Sarrias MJ, Artigas F, Martinez E, Gelpi E, Alvarez E, Udina C et al (1987). Decreased plasma serotonin in melancholic patients: a study with clomipramine. Biol Psychiatry 22: 1429–1438.
Schildkraut JJ (1965). The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry 122: 509–522.
Shaikh MB, Steinberg A, Siegel A (1993). Evidence that Substance P is utilized in medial amygdaloid facilitation of defensive rage behavior in the cat. Brain Res 625: 283–294.
Shirayama Y, Mitsushio H, Takashima M (1996). Reduction of Substance P after chronic antidepressant treatment in the striatum, substantia nigra, and amygdala in rat. Brain Res 739: 70–78.
Smith DW, Hewson L, Fuller P, Williams AR, Wheeldon A, Rupniak NMJ (1999). The substance P antagonist L-760735 inhibits stress-induced NK1 receptor internalization in the basolateral amygdala. Brain Res 848: 90–95.
Takeda Y, Krause JE (1991). Pharmacological and molecular biological studies on the diversity of rat tachykinin NK-2 receptor subtypes in rat CNS, duodenum, vas deferens and urinary bladder. Ann NY Acad Sci 632: 479–482.
Tattersall FD, Rycroft W, Hargreaves RJ, Hill RG (1993). The tachykinin NK-1 receptor antagonist CP-99994 attenuates cisplatin induced emesis in the ferret. Eur J Pharmacol 250: R5–R6.
Tattersall FD, Rycroft W, Hargreaves RJ, Hill RG (1994). Enantioselective inhibition of apomorphine-induced emesis in the ferret by the neurokinin-1 receptor antagonist CP-99994. Neuropharmacology 33: 259–260.
Tignol J, Stoker MJ, Dunbar GC (1992). Paroxetine in the treatment of melancholia and severe depression. Int Clin Psychopharmacol 7: 91–94.
Trindade E, Menon D, Topfer LA, Coloma C (1998). Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. Canadian Medical Association Journal 159: 1245–1252.
Unger Th, Carolus S, Demmert G (1988). Substance P induces a cardiovascular defense reaction in the rat, pharmacological characterization. Circ Res 63: 812–820.
Upadhyaya AK, Pennell I, Cowen PJ, Deakin JF (1991). Blunted growth hormone and prolactin responses to L-tryptophan in depression: a state-dependent abnormality. J Affect Disord 21: 213–218.
Vassout A, Schaub M, Gentsch C, Schilling W, Veenstra S (1994). CGP 49823, a novel NK-1 receptor antagonist: behavioral effects. Neuropeptides 26(Suppl 1): 38.
Willner P (1983). Dopamine and depression: a review of recent evidence. I. Empirical studies. Brain Res Rev 6: 211–246.
Yokota Y, Sasai Y, Tanaka K, Fujiwara T, Tsuchida K, Shigemoto R et al (1989). Molecular characterization of a functional cDNA for rat substance P receptor. J Biol Chem 264: 17649–17652.
Young SN, Smith SE, Pihl R, Ervin FR (1985). Tryptophan depletion causes a rapid lowering of mood in normal males. Psychopharmacology 87: 173–177.
Acknowledgements
This study was supported by a grant from Merck Research Laboratories. We acknowledge Arthur Shedden, MD and Mary Collin, MS for their key contribution to the overall management of the study, Christopher Lines, PhD and Judith Evans, MD for their suggestions on the manuscript and Joan Fleishman for her dedication and hard work.
Reprint requests to: Dr WA Ball, Merck Research Laboratories, 10 Sentry Parkway, Blue Bell, PA 19422
Author information
Authors and Affiliations
Corresponding author
Additional information
NOTE ADDED TO THE PROOF
Since this study was accepted for publication Merck has completed large Phase lll trails with aprepitant, a substance P antagonist similar to L-759274. The results indicated that aprepitant was not more effective than placebo in the treatment of depression
Rights and permissions
About this article
Cite this article
Kramer, M., Winokur, A., Kelsey, J. et al. Demonstration of the Efficacy and Safety of a Novel Substance P (NK1) Receptor Antagonist in Major Depression. Neuropsychopharmacol 29, 385–392 (2004). https://doi.org/10.1038/sj.npp.1300260
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300260
Keywords
This article is cited by
-
The Effect of Blocking Neurokinin-1 Receptor by Aprepitant on the Inflammatory and Apoptosis Pathways in Human Ovarian Cancer Cells
Cell Biochemistry and Biophysics (2022)
-
Cognitive neuropsychological theory of antidepressant action: a modern-day approach to depression and its treatment
Psychopharmacology (2021)
-
Human substance P receptor binding mode of the antagonist drug aprepitant by NMR and crystallography
Nature Communications (2019)
-
The associations of TAC1 gene polymorphisms with major depressive disorder
Molecular & Cellular Toxicology (2019)
-
The association between substance P and white matter integrity in medication-naive patients with major depressive disorder
Scientific Reports (2017)