-
PDF
- Split View
-
Views
-
Cite
Cite
Yazen Alnouti, Curtis D. Klaassen, Tissue Distribution and Ontogeny of Sulfotransferase Enzymes in Mice, Toxicological Sciences, Volume 93, Issue 2, October 2006, Pages 242–255, https://doi.org/10.1093/toxsci/kfl050
Close - Share Icon Share
Abstract
Sulfotransferases (Sults) are phase-II conjugation enzymes that catalyze the transfer of a sulfonate group from 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to target endo and xenobiotics. PAPS is formed from inorganic sulfate by the action of the enzyme PAPS synthase (PAPSs). In the present study, the tissue distribution and developmental changes in the mRNA expression of 11 Sult isozymes and 2 PAPSs isoforms in mice were quantified. Sult1a1, 1b1, 1c1, 1c2, 1d1, 1e1, 2a1/2, 2b1, 3a1, 4a1, 5a1, PAPSs1, and PAPSs2 mRNA expression was quantified in 14 tissues from male and female mice using the branched DNA signal amplification assay. Sult2a1/2 and 3a1 expression were highest in liver; Sult1b1, 2b1, and PAPSs2 in small intestine; Sult1a1 in large intestine; Sult1c2 in stomach; Sult1d1 in kidney; Sult1e1 in placenta; and Sult4a1 in brain. Sult1c1, 5a1, and PAPSs1 were ubiquitously expressed in most tissues. These enzymes demonstrated three different ontogenic expression patterns in liver. Sult1a1, 1c2, 1d1, 2a1/2, and PAPSs2 hepatic expression gradually increased from birth until about 3 weeks of age and then declined somewhat thereafter, Sult1c1 expression was highest before birth and declined after that, and Sult3a1 mRNA expression was very low in fetal livers and remained low until 30 days of age, when expression in females dramatically increased, whereas it never increased in males. The organ-specific distribution of Sults as well as the different expression of the Sults in young animals may affect the pharmacokinetic behavior and organ-specific toxicity of xenobiotics.
The major function of phase-II enzymes is the conjugation of endo- and xenobiotics or their metabolites with endogenous polar moieties to form water-soluble and excretable products. The desired property for the urinary and biliary excretion of metabolites is water solubility, which prevents passive diffusion through cell membranes and thus avoiding reabsorption. Sulfuric acid esters (sulfonates) are formed by enzymatic transfer of the sulfonate group (
The pKa value of most sulfonate conjugates is less than 1, and therefore these molecules at physiological pH are always present in the dissociated water-soluble form (Glatt, 2000). The introduction of a charged moiety to a compound not only dramatically increases their water solubility but also strongly hampers their passive diffusion through cell membranes. Consequently, sulfo-conjugates excreted into urine or bile cannot be passively reabsorbed into the blood stream, unless mediated by a carrier-mediated process. Sulfo-conjugation is also known to directly inactivate pharmacologically active molecules, such as steroid hormones, by preventing their binding to their cognate nuclear receptors (Strott, 2002). Despite their role in inactivation and facilitating the excretion of compounds, sulfo-conjugation can result in the bioactivation of prodrugs to active metabolites, such as in the case of minoxidil (Buhl et al., 1990). Sulfonation can also yield unstable electrophilic species, which are able of forming adducts with DNA, proteins, and other macromolecules, causing mutagenic and carcinogenic effects (Glatt et al., 1998).
In order for sulfonation to occur, inorganic sulfate must be converted to a high-energy form prior to being transferred to an acceptor molecule. 3′-Phosphoadenosine 5′-phosphosulphate (PAPS) is the activated form of sulfate, which functions as the universal donor of the sulfate moiety. PAPS is biosynthesized in two steps: the first reaction is carried out by ATP sulphurylase and results in the formation of adenosine 5′-phosphosulphate (APS), and the second reaction is carried out by APS kinase and results in the formation of PAPS. The two reactions are performed by a single protein in mammals, which is named PAPS synthase (PAPSs) (Lipmann, 1958). Two PAPSs isoenzymes have been cloned from humans and mice, namely, PAPSs1 and PAPSs2. The two isoenzymes differ in their tissue distribution and catalytic activity. PAPSs1 localizes to the nucleus, whereas PAPSs2 localizes to the cytoplasm, except for tissues where PAPSs1 is also expressed (Besset et al., 2000). PAPSs2 has greater catalytic efficiency for PAPS synthesis than PAPSs1 (Fuda et al., 2002).
Based on their subcellular localization, Sults can be classified into two main classes: cytosolic that exists as free proteins in the cytosol and membrane-associated proteins that are bound to the Golgi apparatus. Membrane-associated Sults are involved in post-translational modification of macromolecules, such as carbohydrates, lipids, and proteins. Substrates of this class include glycosaminoglycans, glycoproteins, sphingolipids, and tyrosine residues of proteins (Niehrs et al., 1994). Cytosolic Sults are responsible for the sulfonation of small endogenous and exogenous compounds. Therefore, the cytosolic Sults represent the class relevant to xenobiotics metabolism and disposition.
Based on the amino acid sequence of known mammalian cytosolic Sults, Sults have been categorized into five families, the Sult1 (phenol sulfotransferases, PST), Sult2 (hydroxysteroid Sult), Sult3, Sult4, and Sult5 families. The Sult1 family consists of four subfamily members, 1a (phenol sulfotransferases, PSTs), 1b (dopamine/tyrosine or thyroid hormone Sult), 1c (hydroxylamine or acetylaminofluorene Sult), 1d, and 1e (estrogen Sult). The Sult2 family consists of 2a (dehydrepiandosterone, DHEA Sult) and 2b. Sult2b1 is involved in cholesterol sulfonation (Shimizu et al., 2003). The Sult3 enzymes catalyze the formation of sulfamates, whereas Sult 4 and 5 families have not been adequately characterized. Despite their high sequence similarity, Sult isozymes differ markedly in their substrate specificity, inhibitor sensitivity, and regulation of expression (Yamazoe et al., 1994).
In the present study, the tissue distribution, gender differences, and ontogenic expression of Sult1a1, 1b1, 1c1, 1c2, 1d1, 1e1, 2a1, 2b1, 3a1, 4a1, 5a1, PAPSs1, and PAPSs2 in mice were determined. Most studies in the literature concerning the tissue distribution of Sults have been limited to a few tissues and were not quantitative. These previous studies have primarily been performed in rat and human tissues, with limited data available for mice. Therefore, in the present study, the relative distribution of the various Sults and PAPSs mRNA transcripts as well as the developmental changes in both male and female mice were evaluated. Understanding the tissue-specific expression patterns of Sults may help determine their contribution to the biotransformation of endo- and xenobiotics in various tissues. Furthermore, understanding gender differences and ontogeny of the expression of Sults may help determine the molecular basis for differences in drug disposition between male versus females and newborns versus adults.
MATERIALS AND METHODS
Animals.
Eight-week-old male and female C57BL/6 mice were purchased from Charles River Laboratories Inc. (Wilmington, MA). Animals were housed in a temperature-, light-, and humidity-controlled environment. Mice were fed with Laboratory Rodent Chow W (Harlan Teklad, Madison, WI) ad libitum. Tissues were removed from five mice of each gender, frozen in liquid nitrogen, and stored at − 80°C until mRNA isolation. For the ontogeny study, livers, kidneys, and duodenums from male and female mice were collected at − 2, 0, 5, 10, 15, 22, 30, and 45 days of age (n = 5/gender/age). Male and female pups were pooled at age − 2 and 0 because it was difficult to differentiate their gender.
Total RNA isolation.
Total RNA was isolated using RNA-Bee reagent (Tel-Test, Inc., Friendswood, TX) according to the manufacturer's protocol. Total RNA concentrations were determined spectrophotometrically at 260 nm. Solutions (1 μg/μl) were prepared from stock RNA solutions by dilution with diethyl pyrocarbonate–treated deionized water. Integrity of RNA samples was visualized under ultraviolet light by ethidium bromide fluorescence.
Branched DNA signal amplification analysis.
The mRNA of each Sult in mouse tissues was quantified using the branched DNA (bDNA) signal amplification assay (Quantigene bDNA signal amplification kit; Bayer Corp., Emeryville, CA) with modifications. Gene sequences of interest were accessed from GenBank. Target sequences were analyzed using ProbeDesigner software v1.0 (Bayer Corp.) to design oligonucleotide probe sets (capture, label, and blocker probes). All probes were designed with a melting temperature of 63°C, enabling hybridization conditions to be held constant (i.e., 53°C) during each hybridization step. Each probe developed was submitted to the National Center of Biotechnology Information (Bethesda, MD) by basic local alignment search tool (BLASTn) to ensure minimal cross-reactivity with other known mouse sequences. Oligonucleotides with a high degree of similarity (> 80%) to other mouse gene transcripts were eliminated from the design. The sequences and functions of the probe sets are listed in Table 1. The Sult2a1 and 2a2 isoforms are 96% similar; therefore, the probes designed do not differentiate between the two isoforms. It has been suggested that mouse Sult2a1 and 2a2 are alleles of the same gene. Therefore, our bDNA probe is referred to as Sult2a1/2. Sult2a2 is rarely referred to in the literature. Actually, we found many reports addressing Sult2a1 expression regulation using nonspecific primers or probes, which detect both Sult2a1 and 2a2, or using Sult2a2 primers and refer to it as Sult2a1.
Oligonucleotide Probes Generated for Analysis of Mouse Sult and PAPSs mRNAs Expression by bDNA Signal Amplification Assay
Targeta . | Functionb . | Sequence . | Target . | Function . | Sequence . | |||
|---|---|---|---|---|---|---|---|---|
| Sult1a1 (L_02331) | Sult1c1 (AF_033653) | |||||||
| 617–643 | CE | atagaagagatagagaacagggtgagtTTTTTctcttggaaagaaagt | 1033–1054 | CE | cggaaggtaatagtgctccctgTTTTTctcttggaaagaaagt | |||
| 644–666 | CE | ttgggattctccttcatgtcttcTTTTTctcttggaaagaaagt | 1055–1078 | CE | ccctactgctctcagatctctgtgTTTTTctcttggaaagaaagt | |||
| 667–692 | CE | actctagaatcttcttgatctcccttTTTTTctcttggaaagaaagt | 1168–1193 | CE | ggtctgattaccttttgatgaaatagTTTTTctcttggaaagaaagt | |||
| 514–531 | LE | tcccaggtgcctgggtcaTTTTTaggcataggacccgtgtct | 987–1008 | LE | caaaatcctcactttgtgccacTTTTTaggcataggacccgtgtct | |||
| 555–576 | LE | gacccataggacactttcccatTTTTTaggcataggacccgtgtct | 1102–1126 | LE | ggctcaaatagctaaagcatacagtTTTTTaggcataggacccgtgtct | |||
| 577–597 | LE | tccttcacgtgctggtaccacTTTTTaggcataggacccgtgtct | 1127–1145 | LE | cgcaacgcttagggatggaTTTTTaggcataggacccgtgtct | |||
| 598–616 | LE | gcgtctcagctcccaccacTTTTTaggcataggacccgtgtct | 1146–1167 | LE | aatcatagggcacatagaaccgTTTTTaggcataggacccgtgtct | |||
| 693–711 | LE | ggtagagagcgccccagaaTTTTTaggcataggacccgtgtct | 1194–1218 | LE | agcgtaaccaaaaatttctttagaaTTTTTaggcataggacccgtgtct | |||
| 712–736 | LE | aacaattaaatccacagtctcctcaTTTTTaggcataggacccgtgtct | 1219–1244 | LE | tggttgggtatcaataatattcacatTTTTTaggcataggacccgtgtct | |||
| 782–805 | LE | aacttcagttgggatggttgtgtaTTTTTaggcataggacccgtgtct | 1270–1291 | LE | aacttaccgtttattttggcccTTTTTaggcataggacccgtgtct | |||
| 830–851 | LE | ccccaatggtacctttcctcatTTTTTaggcataggacccgtgtct | 1292–1313 | LE | tttttttttttgggttgtagcgTTTTTaggcataggacccgtgtct | |||
| 532–554 | BL | ccatgaagttctccaagaagctt | 1009–1032 | BL | ccatcttcttccggtagtcttcat | |||
| 737–760 | BL | cattttcttgaaggatgtgtggtg | 1079–1101 | BL | caggacatctaggggtccctcta | |||
| 761–781 | BL | gttagccatggggttctcctt | 1245–1269 | BL | cgagttggtgttgtactgaattgtg | |||
| 806–829 | BL | gaagggataaatagtgtggtccat | ||||||
| Sult1b1 (U_92076) | Sult1c2 (AY_005469) | |||||||
| 340–361 | CE | tccgaggtgatggagttttcttTTTTTctcttggaaagaaagt | 689–711 | CE | cttttccattgatgaaggtttcaTTTTTctcttggaaagaaagt | |||
| 509–530 | CE | tccagatattcttcccaggtgcTTTTTctcttggaaagaaagt | 754–780 | CE | agagaatctgatatttgtctcgaatttTTTTTctcttggaaagaaagt | |||
| 655–678 | CE | gctggctatcttcttgatttctttTTTTTctcttggaaagaaagt | 808–828 | CE | tctggatttcatgctttgggtTTTTTctcttggaaagaaagt | |||
| 288–311 | LE | ccaggaacactcagttccaacattTTTTTaggcataggacccgtgtct | 598–620 | LE | catgcagtctttagcatttcgagTTTTTaggcataggacccgtgtct | |||
| 312–339 | LE | caagagttcaacacctgatattcttattTTTTTaggcataggacccgtgtct | 648–666 | LE | gctctgggagcacctggctTTTTTaggcataggacccgtgtct | |||
| 435–454 | LE | catccttgccatttcgagcaTTTTTaggcataggacccgtgtct | 667–688 | LE | aaatactcatcccaggtgcctgTTTTTaggcataggacccgtgtct | |||
| 455–480 | LE | atcaaaatgataataggagacaggaaTTTTTaggcataggacccgtgtct | 712–732 | LE | caaaccaggatccccaacttaTTTTTaggcataggacccgtgtct | |||
| 531–553 | LE | ccacatttccagctaggaatttcTTTTTaggcataggacccgtgtct | 733–753 | LE | cccaccatcctttcacatggtTTTTTaggcataggacccgtgtct | |||
| 554–577 | LE | catgatcaaaccatgaaccataggTTTTTaggcataggacccgtgtct | 829–849 | LE | tgcccataaactgcatcacctTTTTTaggcataggacccgtgtct | |||
| 704–723 | LE | gacgatcctgtccaaggcctTTTTTaggcataggacccgtgtct | 850–873 | LE | ccaccacatcttcatccaaattctTTTTTaggcataggacccgtgtct | |||
| 768–788 | LE | gctgtgggcagatgggtgtaaTTTTTaggcataggacccgtgtct | 874–900 | LE | caaatgatgtctccaggactattttatTTTTTaggcataggacccgtgtct | |||
| 789–810 | LE | ggacttgctgtggtccatcattTTTTTaggcataggacccgtgtct | 945–967 | LE | atggactggtccaggatagatttTTTTTaggcataggacccgtgtct | |||
| 362–386 | BL | tcgattggaagatgtgtctttatta | 621–647 | BL | catcctgtagaagtggtagtaggaaac | |||
| 387–409 | BL | cccagaaggattttgggagtaga | 781–807 | BL | tcctcttcatatcttcatagaagagga | |||
| 410–434 | BL | aggtaaatcatcttgcacttgttct | 901–926 | BL | tgtcataggattctctttcattttct | |||
| 481–508 | BL | caggaagaggattaatactattcatcag | 927–944 | BL | gggggccgtagaacgatt | |||
| 578–600 | BL | cctcttttcccaccaactcttaa | ||||||
| 601–630 | BL | atagtataagtaaagtaaaggatgctcttc | ||||||
| 631–654 | BL | ctttgggttctgtttcaattcttc | ||||||
| 679–703 | BL | cttcatccaaggtcttgtctagaaa | ||||||
| 724–747 | BL | catcatttcaaaggaggtgtgatg | ||||||
| 748–767 | BL | ttgaccagggggttttcctt | ||||||
| Sult1d1 (U_32371) | Sult2a1/2 (L_27121) | |||||||
| 425–447 | CE | actccatttgttatcccaggaatTTTTTctcttggaaagaaagt | 155–175 | CE | agccagttcgttcctgacttgTTTTTctcttggaaagaaagt | |||
| 494–515 | CE | aaggaagcagctgaacaggaagTTTTTctcttggaaagaaagt | 243–262 | CE | tctatccagggtgagcggtcTTTTTctcttggaaagaaagt | |||
| 612–629 | CE | tgccaggctctgggtggaTTTTTctcttggaaagaaagt | 360–377 | CE | gatcgccttggccttggaTTTTTctcttggaaagaaagt | |||
| 630–652 | CE | tttctctaggaactcttcccaggTTTTTctcttggaaagaaagt | 100–126 | LE | ctttcaccacaaacttattacgaatatTTTTTaggcataggacccgtgtct | |||
| 283–306 | LE | taggtggagatcaaaatgtcatcaTTTTTaggcataggacccgtgtct | 176–201 | LE | tctgaatcaagcatacaatctcattcTTTTTaggcataggacccgtgtct | |||
| 329–355 | LE | gtagatcaaatccagtatttcactgacTTTTTaggcataggacccgtgtct | 263–290 | LE | gattattgcagaatatcctatttcagtcTTTTTaggcataggacccgtgtct | |||
| 356–376 | LE | tttctctgcatccccattgttTTTTTaggcataggacccgtgtct | 291–313 | LE | atgagtcgtggtccttccttattTTTTTaggcataggacccgtgtct | |||
| 377–400 | LE | tttgtagattgcatcccttttacaTTTTTaggcataggacccgtgtct | 378–404 | LE | aatatctctcggatttctcatgagataTTTTTaggcataggacccgtgtct | |||
| 401–424 | LE | tataagctccatgaatggtactcgTTTTTaggcataggacccgtgtct | 430–454 | LE | ggattcttcacaaggtttgtgttacTTTTTaggcataggacccgtgtct | |||
| 448–468 | LE | ggcatgttgttcagcatttcaTTTTTaggcataggacccgtgtct | 500–522 | LE | gctcaaaccatgatccgaatagaTTTTTaggcataggacccgtgtct | |||
| 469–493 | LE | gtgtgttttcactattcgaggagacTTTTTaggcataggacccgtgtct | 523–541 | LE | gacagccagccacggacatTTTTTaggcataggacccgtgtct | |||
| 516–538 | LE | gcagtcatttttccagaatgaggTTTTTaggcataggacccgtgtct | 127–154 | BL | gggtaagttaatatcaacaagtcttctt | |||
| 539–561 | LE | ttccgtgccacataaataatcttTTTTTaggcataggacccgtgtct | 202–221 | BL | ccacttcggatctcccttgg | |||
| 674–695 | LE | catgatcataccagggaccaaaTTTTTaggcataggacccgtgtct | 222–242 | BL | ccaaatgggcacagtttggat | |||
| 307–328 | BL | ccaagttgttccagatttggga | 314–333 | BL | ggatgggaagatgggaggtt | |||
| 562–583 | BL | agaaacaaccacatctttggca | 334–359 | BL | actgaagaaagacttggagaagagat | |||
| 584–611 | BL | tttttgccatttgatagaaataatagta | 405–429 | BL | cccagaaaaagtaaccagacacaag | |||
| 653–673 | BL | gctcacttgtccagccatgaa | 455–477 | BL | caaaataagttccgagtgaccct | |||
| 478–499 | BL | acatttccttggaggaaccatt | ||||||
| Sult1e1 (S_78182) | Sult2b1 (AF_026072) | |||||||
| 387–412 | CE | ccttctcttttaattgttttattccaTTTTTctcttggaaagaaagt | 536–558 | CE | taattgcccagcaatcttagaatTTTTTctcttggaaagaaagt | |||
| 413–439 | CE | ggtgagtttttactattctgggagattTTTTTctcttggaaagaaagt | 578–600 | CE | gaggaaattttgaaggaactggtTTTTTctcttggaaagaaagt | |||
| 732–757 | CE | gctttctctccaggaactctattagcTTTTTctcttggaaagaaagt | 688–708 | CE | gtcctgctgcagctcctcataTTTTTctcttggaaagaaagt | |||
| 260–283 | LE | cttcactaatccaggtggtaccagTTTTTaggcataggacccgtgtct | 512–535 | LE | aataatagagggagaccacgacatTTTTTaggcataggacccgtgtct | |||
| 284–313 | LE | catcaccttctttatagatcatatacacaaTTTTTaggcataggacccgtgtct | 559–577 | LE | cgggtgtaccagggtccttTTTTTaggcataggacccgtgtct | |||
| 314–334 | LE | catcctccttgcatttttccaTTTTTaggcataggacccgtgtct | 601–622 | LE | agccaaactgcacttctcctttTTTTTaggcataggacccgtgtct | |||
| 335–361 | LE | ccaaataaggtattctgttaaaaattgTTTTTaggcataggacccgtgtct | 623–643 | LE | ccttgatgtggtcaaaccaggTTTTTaggcataggacccgtgtct | |||
| 362–386 | LE | tttattaggtcttcgtttctgcactTTTTTaggcataggacccgtgtct | 644–662 | LE | ttctgcatccggatccagcTTTTTaggcataggacccgtgtct | |||
| 459–481 | LE | aattcttttcccaaaatgatgctTTTTTaggcataggacccgtgtct | 709–725 | LE | tgcacggagcctcgcagTTTTTaggcataggacccgtgtct | |||
| 582–605 | LE | tgcataaatttctccacaaattcaTTTTTaggcataggacccgtgtct | 726–746 | LE | cccaggaactcacagatgcgtTTTTTaggcataggacccgtgtct | |||
| 627–650 | LE | caagctttcacatgatcataccagTTTTTaggcataggacccgtgtct | 765–783 | LE | caccacagagctcagggccTTTTTaggcataggacccgtgtct | |||
| 758–776 | LE | tccacaagctctgccgaggTTTTTaggcataggacccgtgtct | 803–823 | LE | acatggtattggccttcatggTTTTTaggcataggacccgtgtct | |||
| 440–458 | BL | ggaaggaccttgggtggca | 663–687 | BL | ggtgataaacaggaagttctcttgg | |||
| 482–503 | BL | cggcaaagatagatcatcttgc | 747–764 | BL | tcttcacccagtggccgg | |||
| 504–522 | BL | ggcgacatctttggcgttc | 784–802 | BL | cagcaaaggctgagtgggc | |||
| 523–553 | BL | ttatcattagcaaaaagtagtaataagaaac | ||||||
| 554–581 | BL | gaaaaagatttaggatttggataactag | ||||||
| 606–626 | BL | gaaccatacggaacttgccct | ||||||
| 651–674 | BL | cgtgaattcttactcttttcccac | ||||||
| 675–703 | BL | tcatgtcttcatagaacataaataaaaca | ||||||
| 704–731 | BL | tttacaacttctcttctgatatcctctt | ||||||
| Sult3a1 (AF026075) | Sult5a1 (AF_026074) | |||||||
| 211–231 | CE | aaaggatctgctgggtccagaTTTTTctcttggaaagaaagt | 555–574 | CE | gcctgtgccctcgagaaactTTTTTctcttggaaagaaagt | |||
| 256–278 | CE | ttcgatgttttcagttctgttccTTTTTctcttggaaagaaagt | 719–739 | CE | gatatcctcctcttttggcccTTTTTctcttggaaagaaagt | |||
| 401–425 | CE | catataaagaattttggcttttttgTTTTTctcttggaaagaaagt | 762–786 | CE | actatgttgctctgactcatgaaggTTTTTctcttggaaagaaagt | |||
| 46–73 | LE | ttattgtccataatttgttacctctagtTTTTTaggcataggacccgtgtct | 595–612 | LE | agccaccccttcacgtggTTTTTaggcataggacccgtgtct | |||
| 132–161 | LE | ttcataattttctatattttccactacttcTTTTTaggcataggacccgtgtct | 613–634 | LE | agttaggtccttctgcaggctcTTTTTaggcataggacccgtgtct | |||
| 232–255 | LE | gatgaccctcaaaataaatcaaggTTTTTaggcataggacccgtgtct | 660–678 | LE | aagcgaggttcctggtgcaTTTTTaggcataggacccgtgtct | |||
| 333–351 | LE | tgcgaggtgatggcattttTTTTTaggcataggacccgtgtct | 740–761 | LE | aaaagctgctgtgttccaggatTTTTTaggcataggacccgtgtct | |||
| 426–452 | LE | gatcaaaacatctttaggatttctgtaTTTTTaggcataggacccgtgtct | 809–831 | LE | ccctcgctctggtctatgatctcTTTTTaggcataggacccgtgtct | |||
| 533–554 | LE | aaggcttcctaccacatctccaTTTTTaggcataggacccgtgtct | 855–875 | LE | agtattccctccagttccccaTTTTTaggcataggacccgtgtct | |||
| 74–100 | BL | cctttgaagttaagcaaatattcatct | 876–900 | LE | aacttctcattcagctcaggagtaaTTTTTaggcataggacccgtgtct | |||
| 101–131 | BL | cattttaactaaagttttctgaaaattatag | 923–942 | LE | caaaggccagagtcacccatTTTTTaggcataggacccgtgtct | |||
| 162–186 | BL | caatgaagatgtcatcatctcgaat | 575–594 | BL | tcaaaccaggagccgaagaa | |||
| 187–210 | BL | tggtaccagactttggatatgtga | 635–659 | BL | gctcctcataggtgacaaaaaacaa | |||
| 279–304 | BL | tcaaaaaatggtgctctatctattgt | 679–704 | BL | ggaattcacttaacttgcggatagta | |||
| 305–332 | BL | ggcatagtctaatttgtgaatattgtac | 705–718 | BL | cagggggcgcccca | |||
| 352–375 | BL | aatatggaatgtgggaactgaaga | 787–808 | BL | cttggacagcaggctgtagttg | |||
| 376–400 | BL | tccttgagaccttttggtactaagt | 832–854 | BL | caacacctttcctgaaaaacttg | |||
| 453–478 | BL | atcaaatttgagaaatgaaaatagga | 901–922 | BL | cttggactggtagacagcgttg | |||
| 479–504 | BL | cagtgtctggattttgaaatataagc | ||||||
| 505–532 | BL | tctagaaatgtttgcataaaactttcta | ||||||
| Sult4a1 (AF_059257) | PAPSs1 (U_34883) | |||||||
| 237–252 | CE | gggtcggcaccctggcTTTTTctcttggaaagaaagt | 344–366 | CE | gcgaacgttctcttctctgtcctTTTTTctcttggaaagaaagt | |||
| 373–390 | CE | tcagagggcaggaagcggTTTTTctcttggaaagaaagt | 455–475 | CE | cctcatgaatctgccttgcgtTTTTTctcttggaaagaaagt | |||
| 496–516 | CE | cagaactcctggaaggtgcctTTTTTctcttggaaagaaagt | 578–598 | CE | cgatgccagtgaagccttttaTTTTTctcttggaaagaaagt | |||
| 253–273 | LE | ttcatcaggccgatttcatcaTTTTTaggcataggacccgtgtct | 794–816 | CE | tttattgattttcagggctggtaTTTTTctcttggaaagaaagt | |||
| 274–292 | LE | cggcagctgctcgtcaatgTTTTTaggcataggacccgtgtct | 367–384 | LE | cgccacctcagctatgcgTTTTTaggcataggacccgtgtct | |||
| 293–312 | LE | ggctgtgggtactccagcacTTTTTaggcataggacccgtgtct | 385–405 | LE | gccagcatctgcaaacagcttTTTTTaggcataggacccgtgtct | |||
| 313–332 | LE | ccttgatgatgtccagccccTTTTTaggcataggacccgtgtct | 433–454 | LE | tgttgcgatcctgtgtgtaaggTTTTTaggcataggacccgtgtct | |||
| 411–431 | LE | tgcgagccatgtagatgacctTTTTTaggcataggacccgtgtct | 496–518 | LE | agaggagcatcgacaaaaacttcTTTTTaggcataggacccgtgtct | |||
| 476–495 | LE | cggtagctcatggtccgcagTTTTTaggcataggacccgtgtct | 519–539 | LE | tccctctgctcacagacatgcTTTTTaggcataggacccgtgtct | |||
| 517–538 | LE | tttgtcattcatgaacctccgaTTTTTaggcataggacccgtgtct | 599–622 | LE | cctcgggtttctcatactcagaatTTTTTaggcataggacccgtgtct | |||
| 539–556 | LE | ccaggagccgtagcccagTTTTTaggcataggacccgtgtct | 640–660 | LE | gacgtcacaggaatccgttttTTTTTaggcataggacccgtgtct | |||
| 557–579 | LE | cagaattcctgaacatgctcaaaTTTTTaggcataggacccgtgtct | 661–679 | LE | cctgctggacgcagtcgttTTTTTaggcataggacccgtgtct | |||
| 580–599 | LE | tggcatccattcgatgttccTTTTTaggcataggacccgtgtct | 702–721 | LE | catccacagggacgatgtccTTTTTaggcataggacccgtgtct | |||
| 333–352 | BL | gaggcggggagatgtcagtt | 773–793 | LE | aggcttctgcatcagttttggTTTTTaggcataggacccgtgtct | |||
| 353–372 | BL | tacgggaggtggctcttgat | 817–836 | LE | tgcacccactgcatatccacTTTTTaggcataggacccgtgtct | |||
| 391–410 | BL | tggagtccccattgtggagg | 837–856 | LE | cccaaccttctgccaaaaccTTTTTaggcataggacccgtgtct | |||
| 432–452 | BL | ataccaccaagtccttggggt | 406–432 | BL | cgatataaagctggtgatacacactaa | |||
| 453–475 | BL | cgagcggtggaactgatagtaag | 476–495 | BL | aaagaagggcaagcttgcac | |||
| 540–562 | BL | ccttcttgtagaggcctttgaca | ||||||
| 563–577 | BL | tctcccctgcccgcg | ||||||
| 623–639 | BL | cagcaccagctccgggg | ||||||
| 680–701 | BL | cgttcctgaagaagctccacaa | ||||||
| 722–748 | BL | catatagctctttcacttcataggaag | ||||||
| PAPSs2 (AF_052453) | ||||||||
| 238–256 | CE | tcggaatcctcccctggttTTTTTctcttggaaagaaagt | ||||||
| 412–432 | CE | atattctcttctcggtcccccTTTTTctcttggaaagaaagt | ||||||
| 544–564 | CE | aagaacgggagtcctgctgatTTTTTctcttggaaagaaagt | ||||||
| 612–635 | CE | gggctcgtttgtagagtccttttaTTTTTctcttggaaagaaagt | ||||||
| 257–278 | LE | ctgttagccacacggtacatccTTTTTaggcataggacccgtgtct | ||||||
| 346–367 | LE | cccatccagagagtaacatgggTTTTTaggcataggacccgtgtct | ||||||
| 368–387 | LE | aggccatgacggacattgtcTTTTTaggcataggacccgtgtct | ||||||
| 504–525 | LE | ttctcacgatcctttgcaaaagTTTTTaggcataggacccgtgtct | ||||||
| 526–543 | LE | tcgtggatttttcgggcaTTTTTaggcataggacccgtgtct | ||||||
| 587–611 | LE | cgtctcggctttcacagatatttaaTTTTTaggcataggacccgtgtct | ||||||
| 636–658 | LE | aaaccctttaatctctcctgctcTTTTTaggcataggacccgtgtct | ||||||
| 659–681 | LE | tcatagtcagaatcgatgcctgtTTTTTaggcataggacccgtgtct | ||||||
| 279–299 | BL | ttttcccagcaccagagagac | ||||||
| 300–321 | BL | tccaaagcaaagcttatggttg | ||||||
| 322–345 | BL | atggcgtgagatacaaggtactct | ||||||
| 388–411 | BL | gcagagaatcccaggttcttatta | ||||||
| 433–446 | BL | ccgcgatccggcgg | ||||||
| 447–465 | BL | gcaaagagcctggccacct | ||||||
| 466–480 | BL | accaggccggcgtcg | ||||||
| 481–503 | BL | gagagataaagctggtgatgcaa | ||||||
| 565–586 | BL | aggcgcatctacaaagatctca | ||||||
Targeta . | Functionb . | Sequence . | Target . | Function . | Sequence . | |||
|---|---|---|---|---|---|---|---|---|
| Sult1a1 (L_02331) | Sult1c1 (AF_033653) | |||||||
| 617–643 | CE | atagaagagatagagaacagggtgagtTTTTTctcttggaaagaaagt | 1033–1054 | CE | cggaaggtaatagtgctccctgTTTTTctcttggaaagaaagt | |||
| 644–666 | CE | ttgggattctccttcatgtcttcTTTTTctcttggaaagaaagt | 1055–1078 | CE | ccctactgctctcagatctctgtgTTTTTctcttggaaagaaagt | |||
| 667–692 | CE | actctagaatcttcttgatctcccttTTTTTctcttggaaagaaagt | 1168–1193 | CE | ggtctgattaccttttgatgaaatagTTTTTctcttggaaagaaagt | |||
| 514–531 | LE | tcccaggtgcctgggtcaTTTTTaggcataggacccgtgtct | 987–1008 | LE | caaaatcctcactttgtgccacTTTTTaggcataggacccgtgtct | |||
| 555–576 | LE | gacccataggacactttcccatTTTTTaggcataggacccgtgtct | 1102–1126 | LE | ggctcaaatagctaaagcatacagtTTTTTaggcataggacccgtgtct | |||
| 577–597 | LE | tccttcacgtgctggtaccacTTTTTaggcataggacccgtgtct | 1127–1145 | LE | cgcaacgcttagggatggaTTTTTaggcataggacccgtgtct | |||
| 598–616 | LE | gcgtctcagctcccaccacTTTTTaggcataggacccgtgtct | 1146–1167 | LE | aatcatagggcacatagaaccgTTTTTaggcataggacccgtgtct | |||
| 693–711 | LE | ggtagagagcgccccagaaTTTTTaggcataggacccgtgtct | 1194–1218 | LE | agcgtaaccaaaaatttctttagaaTTTTTaggcataggacccgtgtct | |||
| 712–736 | LE | aacaattaaatccacagtctcctcaTTTTTaggcataggacccgtgtct | 1219–1244 | LE | tggttgggtatcaataatattcacatTTTTTaggcataggacccgtgtct | |||
| 782–805 | LE | aacttcagttgggatggttgtgtaTTTTTaggcataggacccgtgtct | 1270–1291 | LE | aacttaccgtttattttggcccTTTTTaggcataggacccgtgtct | |||
| 830–851 | LE | ccccaatggtacctttcctcatTTTTTaggcataggacccgtgtct | 1292–1313 | LE | tttttttttttgggttgtagcgTTTTTaggcataggacccgtgtct | |||
| 532–554 | BL | ccatgaagttctccaagaagctt | 1009–1032 | BL | ccatcttcttccggtagtcttcat | |||
| 737–760 | BL | cattttcttgaaggatgtgtggtg | 1079–1101 | BL | caggacatctaggggtccctcta | |||
| 761–781 | BL | gttagccatggggttctcctt | 1245–1269 | BL | cgagttggtgttgtactgaattgtg | |||
| 806–829 | BL | gaagggataaatagtgtggtccat | ||||||
| Sult1b1 (U_92076) | Sult1c2 (AY_005469) | |||||||
| 340–361 | CE | tccgaggtgatggagttttcttTTTTTctcttggaaagaaagt | 689–711 | CE | cttttccattgatgaaggtttcaTTTTTctcttggaaagaaagt | |||
| 509–530 | CE | tccagatattcttcccaggtgcTTTTTctcttggaaagaaagt | 754–780 | CE | agagaatctgatatttgtctcgaatttTTTTTctcttggaaagaaagt | |||
| 655–678 | CE | gctggctatcttcttgatttctttTTTTTctcttggaaagaaagt | 808–828 | CE | tctggatttcatgctttgggtTTTTTctcttggaaagaaagt | |||
| 288–311 | LE | ccaggaacactcagttccaacattTTTTTaggcataggacccgtgtct | 598–620 | LE | catgcagtctttagcatttcgagTTTTTaggcataggacccgtgtct | |||
| 312–339 | LE | caagagttcaacacctgatattcttattTTTTTaggcataggacccgtgtct | 648–666 | LE | gctctgggagcacctggctTTTTTaggcataggacccgtgtct | |||
| 435–454 | LE | catccttgccatttcgagcaTTTTTaggcataggacccgtgtct | 667–688 | LE | aaatactcatcccaggtgcctgTTTTTaggcataggacccgtgtct | |||
| 455–480 | LE | atcaaaatgataataggagacaggaaTTTTTaggcataggacccgtgtct | 712–732 | LE | caaaccaggatccccaacttaTTTTTaggcataggacccgtgtct | |||
| 531–553 | LE | ccacatttccagctaggaatttcTTTTTaggcataggacccgtgtct | 733–753 | LE | cccaccatcctttcacatggtTTTTTaggcataggacccgtgtct | |||
| 554–577 | LE | catgatcaaaccatgaaccataggTTTTTaggcataggacccgtgtct | 829–849 | LE | tgcccataaactgcatcacctTTTTTaggcataggacccgtgtct | |||
| 704–723 | LE | gacgatcctgtccaaggcctTTTTTaggcataggacccgtgtct | 850–873 | LE | ccaccacatcttcatccaaattctTTTTTaggcataggacccgtgtct | |||
| 768–788 | LE | gctgtgggcagatgggtgtaaTTTTTaggcataggacccgtgtct | 874–900 | LE | caaatgatgtctccaggactattttatTTTTTaggcataggacccgtgtct | |||
| 789–810 | LE | ggacttgctgtggtccatcattTTTTTaggcataggacccgtgtct | 945–967 | LE | atggactggtccaggatagatttTTTTTaggcataggacccgtgtct | |||
| 362–386 | BL | tcgattggaagatgtgtctttatta | 621–647 | BL | catcctgtagaagtggtagtaggaaac | |||
| 387–409 | BL | cccagaaggattttgggagtaga | 781–807 | BL | tcctcttcatatcttcatagaagagga | |||
| 410–434 | BL | aggtaaatcatcttgcacttgttct | 901–926 | BL | tgtcataggattctctttcattttct | |||
| 481–508 | BL | caggaagaggattaatactattcatcag | 927–944 | BL | gggggccgtagaacgatt | |||
| 578–600 | BL | cctcttttcccaccaactcttaa | ||||||
| 601–630 | BL | atagtataagtaaagtaaaggatgctcttc | ||||||
| 631–654 | BL | ctttgggttctgtttcaattcttc | ||||||
| 679–703 | BL | cttcatccaaggtcttgtctagaaa | ||||||
| 724–747 | BL | catcatttcaaaggaggtgtgatg | ||||||
| 748–767 | BL | ttgaccagggggttttcctt | ||||||
| Sult1d1 (U_32371) | Sult2a1/2 (L_27121) | |||||||
| 425–447 | CE | actccatttgttatcccaggaatTTTTTctcttggaaagaaagt | 155–175 | CE | agccagttcgttcctgacttgTTTTTctcttggaaagaaagt | |||
| 494–515 | CE | aaggaagcagctgaacaggaagTTTTTctcttggaaagaaagt | 243–262 | CE | tctatccagggtgagcggtcTTTTTctcttggaaagaaagt | |||
| 612–629 | CE | tgccaggctctgggtggaTTTTTctcttggaaagaaagt | 360–377 | CE | gatcgccttggccttggaTTTTTctcttggaaagaaagt | |||
| 630–652 | CE | tttctctaggaactcttcccaggTTTTTctcttggaaagaaagt | 100–126 | LE | ctttcaccacaaacttattacgaatatTTTTTaggcataggacccgtgtct | |||
| 283–306 | LE | taggtggagatcaaaatgtcatcaTTTTTaggcataggacccgtgtct | 176–201 | LE | tctgaatcaagcatacaatctcattcTTTTTaggcataggacccgtgtct | |||
| 329–355 | LE | gtagatcaaatccagtatttcactgacTTTTTaggcataggacccgtgtct | 263–290 | LE | gattattgcagaatatcctatttcagtcTTTTTaggcataggacccgtgtct | |||
| 356–376 | LE | tttctctgcatccccattgttTTTTTaggcataggacccgtgtct | 291–313 | LE | atgagtcgtggtccttccttattTTTTTaggcataggacccgtgtct | |||
| 377–400 | LE | tttgtagattgcatcccttttacaTTTTTaggcataggacccgtgtct | 378–404 | LE | aatatctctcggatttctcatgagataTTTTTaggcataggacccgtgtct | |||
| 401–424 | LE | tataagctccatgaatggtactcgTTTTTaggcataggacccgtgtct | 430–454 | LE | ggattcttcacaaggtttgtgttacTTTTTaggcataggacccgtgtct | |||
| 448–468 | LE | ggcatgttgttcagcatttcaTTTTTaggcataggacccgtgtct | 500–522 | LE | gctcaaaccatgatccgaatagaTTTTTaggcataggacccgtgtct | |||
| 469–493 | LE | gtgtgttttcactattcgaggagacTTTTTaggcataggacccgtgtct | 523–541 | LE | gacagccagccacggacatTTTTTaggcataggacccgtgtct | |||
| 516–538 | LE | gcagtcatttttccagaatgaggTTTTTaggcataggacccgtgtct | 127–154 | BL | gggtaagttaatatcaacaagtcttctt | |||
| 539–561 | LE | ttccgtgccacataaataatcttTTTTTaggcataggacccgtgtct | 202–221 | BL | ccacttcggatctcccttgg | |||
| 674–695 | LE | catgatcataccagggaccaaaTTTTTaggcataggacccgtgtct | 222–242 | BL | ccaaatgggcacagtttggat | |||
| 307–328 | BL | ccaagttgttccagatttggga | 314–333 | BL | ggatgggaagatgggaggtt | |||
| 562–583 | BL | agaaacaaccacatctttggca | 334–359 | BL | actgaagaaagacttggagaagagat | |||
| 584–611 | BL | tttttgccatttgatagaaataatagta | 405–429 | BL | cccagaaaaagtaaccagacacaag | |||
| 653–673 | BL | gctcacttgtccagccatgaa | 455–477 | BL | caaaataagttccgagtgaccct | |||
| 478–499 | BL | acatttccttggaggaaccatt | ||||||
| Sult1e1 (S_78182) | Sult2b1 (AF_026072) | |||||||
| 387–412 | CE | ccttctcttttaattgttttattccaTTTTTctcttggaaagaaagt | 536–558 | CE | taattgcccagcaatcttagaatTTTTTctcttggaaagaaagt | |||
| 413–439 | CE | ggtgagtttttactattctgggagattTTTTTctcttggaaagaaagt | 578–600 | CE | gaggaaattttgaaggaactggtTTTTTctcttggaaagaaagt | |||
| 732–757 | CE | gctttctctccaggaactctattagcTTTTTctcttggaaagaaagt | 688–708 | CE | gtcctgctgcagctcctcataTTTTTctcttggaaagaaagt | |||
| 260–283 | LE | cttcactaatccaggtggtaccagTTTTTaggcataggacccgtgtct | 512–535 | LE | aataatagagggagaccacgacatTTTTTaggcataggacccgtgtct | |||
| 284–313 | LE | catcaccttctttatagatcatatacacaaTTTTTaggcataggacccgtgtct | 559–577 | LE | cgggtgtaccagggtccttTTTTTaggcataggacccgtgtct | |||
| 314–334 | LE | catcctccttgcatttttccaTTTTTaggcataggacccgtgtct | 601–622 | LE | agccaaactgcacttctcctttTTTTTaggcataggacccgtgtct | |||
| 335–361 | LE | ccaaataaggtattctgttaaaaattgTTTTTaggcataggacccgtgtct | 623–643 | LE | ccttgatgtggtcaaaccaggTTTTTaggcataggacccgtgtct | |||
| 362–386 | LE | tttattaggtcttcgtttctgcactTTTTTaggcataggacccgtgtct | 644–662 | LE | ttctgcatccggatccagcTTTTTaggcataggacccgtgtct | |||
| 459–481 | LE | aattcttttcccaaaatgatgctTTTTTaggcataggacccgtgtct | 709–725 | LE | tgcacggagcctcgcagTTTTTaggcataggacccgtgtct | |||
| 582–605 | LE | tgcataaatttctccacaaattcaTTTTTaggcataggacccgtgtct | 726–746 | LE | cccaggaactcacagatgcgtTTTTTaggcataggacccgtgtct | |||
| 627–650 | LE | caagctttcacatgatcataccagTTTTTaggcataggacccgtgtct | 765–783 | LE | caccacagagctcagggccTTTTTaggcataggacccgtgtct | |||
| 758–776 | LE | tccacaagctctgccgaggTTTTTaggcataggacccgtgtct | 803–823 | LE | acatggtattggccttcatggTTTTTaggcataggacccgtgtct | |||
| 440–458 | BL | ggaaggaccttgggtggca | 663–687 | BL | ggtgataaacaggaagttctcttgg | |||
| 482–503 | BL | cggcaaagatagatcatcttgc | 747–764 | BL | tcttcacccagtggccgg | |||
| 504–522 | BL | ggcgacatctttggcgttc | 784–802 | BL | cagcaaaggctgagtgggc | |||
| 523–553 | BL | ttatcattagcaaaaagtagtaataagaaac | ||||||
| 554–581 | BL | gaaaaagatttaggatttggataactag | ||||||
| 606–626 | BL | gaaccatacggaacttgccct | ||||||
| 651–674 | BL | cgtgaattcttactcttttcccac | ||||||
| 675–703 | BL | tcatgtcttcatagaacataaataaaaca | ||||||
| 704–731 | BL | tttacaacttctcttctgatatcctctt | ||||||
| Sult3a1 (AF026075) | Sult5a1 (AF_026074) | |||||||
| 211–231 | CE | aaaggatctgctgggtccagaTTTTTctcttggaaagaaagt | 555–574 | CE | gcctgtgccctcgagaaactTTTTTctcttggaaagaaagt | |||
| 256–278 | CE | ttcgatgttttcagttctgttccTTTTTctcttggaaagaaagt | 719–739 | CE | gatatcctcctcttttggcccTTTTTctcttggaaagaaagt | |||
| 401–425 | CE | catataaagaattttggcttttttgTTTTTctcttggaaagaaagt | 762–786 | CE | actatgttgctctgactcatgaaggTTTTTctcttggaaagaaagt | |||
| 46–73 | LE | ttattgtccataatttgttacctctagtTTTTTaggcataggacccgtgtct | 595–612 | LE | agccaccccttcacgtggTTTTTaggcataggacccgtgtct | |||
| 132–161 | LE | ttcataattttctatattttccactacttcTTTTTaggcataggacccgtgtct | 613–634 | LE | agttaggtccttctgcaggctcTTTTTaggcataggacccgtgtct | |||
| 232–255 | LE | gatgaccctcaaaataaatcaaggTTTTTaggcataggacccgtgtct | 660–678 | LE | aagcgaggttcctggtgcaTTTTTaggcataggacccgtgtct | |||
| 333–351 | LE | tgcgaggtgatggcattttTTTTTaggcataggacccgtgtct | 740–761 | LE | aaaagctgctgtgttccaggatTTTTTaggcataggacccgtgtct | |||
| 426–452 | LE | gatcaaaacatctttaggatttctgtaTTTTTaggcataggacccgtgtct | 809–831 | LE | ccctcgctctggtctatgatctcTTTTTaggcataggacccgtgtct | |||
| 533–554 | LE | aaggcttcctaccacatctccaTTTTTaggcataggacccgtgtct | 855–875 | LE | agtattccctccagttccccaTTTTTaggcataggacccgtgtct | |||
| 74–100 | BL | cctttgaagttaagcaaatattcatct | 876–900 | LE | aacttctcattcagctcaggagtaaTTTTTaggcataggacccgtgtct | |||
| 101–131 | BL | cattttaactaaagttttctgaaaattatag | 923–942 | LE | caaaggccagagtcacccatTTTTTaggcataggacccgtgtct | |||
| 162–186 | BL | caatgaagatgtcatcatctcgaat | 575–594 | BL | tcaaaccaggagccgaagaa | |||
| 187–210 | BL | tggtaccagactttggatatgtga | 635–659 | BL | gctcctcataggtgacaaaaaacaa | |||
| 279–304 | BL | tcaaaaaatggtgctctatctattgt | 679–704 | BL | ggaattcacttaacttgcggatagta | |||
| 305–332 | BL | ggcatagtctaatttgtgaatattgtac | 705–718 | BL | cagggggcgcccca | |||
| 352–375 | BL | aatatggaatgtgggaactgaaga | 787–808 | BL | cttggacagcaggctgtagttg | |||
| 376–400 | BL | tccttgagaccttttggtactaagt | 832–854 | BL | caacacctttcctgaaaaacttg | |||
| 453–478 | BL | atcaaatttgagaaatgaaaatagga | 901–922 | BL | cttggactggtagacagcgttg | |||
| 479–504 | BL | cagtgtctggattttgaaatataagc | ||||||
| 505–532 | BL | tctagaaatgtttgcataaaactttcta | ||||||
| Sult4a1 (AF_059257) | PAPSs1 (U_34883) | |||||||
| 237–252 | CE | gggtcggcaccctggcTTTTTctcttggaaagaaagt | 344–366 | CE | gcgaacgttctcttctctgtcctTTTTTctcttggaaagaaagt | |||
| 373–390 | CE | tcagagggcaggaagcggTTTTTctcttggaaagaaagt | 455–475 | CE | cctcatgaatctgccttgcgtTTTTTctcttggaaagaaagt | |||
| 496–516 | CE | cagaactcctggaaggtgcctTTTTTctcttggaaagaaagt | 578–598 | CE | cgatgccagtgaagccttttaTTTTTctcttggaaagaaagt | |||
| 253–273 | LE | ttcatcaggccgatttcatcaTTTTTaggcataggacccgtgtct | 794–816 | CE | tttattgattttcagggctggtaTTTTTctcttggaaagaaagt | |||
| 274–292 | LE | cggcagctgctcgtcaatgTTTTTaggcataggacccgtgtct | 367–384 | LE | cgccacctcagctatgcgTTTTTaggcataggacccgtgtct | |||
| 293–312 | LE | ggctgtgggtactccagcacTTTTTaggcataggacccgtgtct | 385–405 | LE | gccagcatctgcaaacagcttTTTTTaggcataggacccgtgtct | |||
| 313–332 | LE | ccttgatgatgtccagccccTTTTTaggcataggacccgtgtct | 433–454 | LE | tgttgcgatcctgtgtgtaaggTTTTTaggcataggacccgtgtct | |||
| 411–431 | LE | tgcgagccatgtagatgacctTTTTTaggcataggacccgtgtct | 496–518 | LE | agaggagcatcgacaaaaacttcTTTTTaggcataggacccgtgtct | |||
| 476–495 | LE | cggtagctcatggtccgcagTTTTTaggcataggacccgtgtct | 519–539 | LE | tccctctgctcacagacatgcTTTTTaggcataggacccgtgtct | |||
| 517–538 | LE | tttgtcattcatgaacctccgaTTTTTaggcataggacccgtgtct | 599–622 | LE | cctcgggtttctcatactcagaatTTTTTaggcataggacccgtgtct | |||
| 539–556 | LE | ccaggagccgtagcccagTTTTTaggcataggacccgtgtct | 640–660 | LE | gacgtcacaggaatccgttttTTTTTaggcataggacccgtgtct | |||
| 557–579 | LE | cagaattcctgaacatgctcaaaTTTTTaggcataggacccgtgtct | 661–679 | LE | cctgctggacgcagtcgttTTTTTaggcataggacccgtgtct | |||
| 580–599 | LE | tggcatccattcgatgttccTTTTTaggcataggacccgtgtct | 702–721 | LE | catccacagggacgatgtccTTTTTaggcataggacccgtgtct | |||
| 333–352 | BL | gaggcggggagatgtcagtt | 773–793 | LE | aggcttctgcatcagttttggTTTTTaggcataggacccgtgtct | |||
| 353–372 | BL | tacgggaggtggctcttgat | 817–836 | LE | tgcacccactgcatatccacTTTTTaggcataggacccgtgtct | |||
| 391–410 | BL | tggagtccccattgtggagg | 837–856 | LE | cccaaccttctgccaaaaccTTTTTaggcataggacccgtgtct | |||
| 432–452 | BL | ataccaccaagtccttggggt | 406–432 | BL | cgatataaagctggtgatacacactaa | |||
| 453–475 | BL | cgagcggtggaactgatagtaag | 476–495 | BL | aaagaagggcaagcttgcac | |||
| 540–562 | BL | ccttcttgtagaggcctttgaca | ||||||
| 563–577 | BL | tctcccctgcccgcg | ||||||
| 623–639 | BL | cagcaccagctccgggg | ||||||
| 680–701 | BL | cgttcctgaagaagctccacaa | ||||||
| 722–748 | BL | catatagctctttcacttcataggaag | ||||||
| PAPSs2 (AF_052453) | ||||||||
| 238–256 | CE | tcggaatcctcccctggttTTTTTctcttggaaagaaagt | ||||||
| 412–432 | CE | atattctcttctcggtcccccTTTTTctcttggaaagaaagt | ||||||
| 544–564 | CE | aagaacgggagtcctgctgatTTTTTctcttggaaagaaagt | ||||||
| 612–635 | CE | gggctcgtttgtagagtccttttaTTTTTctcttggaaagaaagt | ||||||
| 257–278 | LE | ctgttagccacacggtacatccTTTTTaggcataggacccgtgtct | ||||||
| 346–367 | LE | cccatccagagagtaacatgggTTTTTaggcataggacccgtgtct | ||||||
| 368–387 | LE | aggccatgacggacattgtcTTTTTaggcataggacccgtgtct | ||||||
| 504–525 | LE | ttctcacgatcctttgcaaaagTTTTTaggcataggacccgtgtct | ||||||
| 526–543 | LE | tcgtggatttttcgggcaTTTTTaggcataggacccgtgtct | ||||||
| 587–611 | LE | cgtctcggctttcacagatatttaaTTTTTaggcataggacccgtgtct | ||||||
| 636–658 | LE | aaaccctttaatctctcctgctcTTTTTaggcataggacccgtgtct | ||||||
| 659–681 | LE | tcatagtcagaatcgatgcctgtTTTTTaggcataggacccgtgtct | ||||||
| 279–299 | BL | ttttcccagcaccagagagac | ||||||
| 300–321 | BL | tccaaagcaaagcttatggttg | ||||||
| 322–345 | BL | atggcgtgagatacaaggtactct | ||||||
| 388–411 | BL | gcagagaatcccaggttcttatta | ||||||
| 433–446 | BL | ccgcgatccggcgg | ||||||
| 447–465 | BL | gcaaagagcctggccacct | ||||||
| 466–480 | BL | accaggccggcgtcg | ||||||
| 481–503 | BL | gagagataaagctggtgatgcaa | ||||||
| 565–586 | BL | aggcgcatctacaaagatctca | ||||||
CE, capture extender; LE, label extender; BL, blocker.
Target refers to the sequence of the mRNA transcript as enumerated in the GenBank file.
Function refers to the utility of the oligonucleotide probe in the bDNA assay.
Oligonucleotide Probes Generated for Analysis of Mouse Sult and PAPSs mRNAs Expression by bDNA Signal Amplification Assay
Targeta . | Functionb . | Sequence . | Target . | Function . | Sequence . | |||
|---|---|---|---|---|---|---|---|---|
| Sult1a1 (L_02331) | Sult1c1 (AF_033653) | |||||||
| 617–643 | CE | atagaagagatagagaacagggtgagtTTTTTctcttggaaagaaagt | 1033–1054 | CE | cggaaggtaatagtgctccctgTTTTTctcttggaaagaaagt | |||
| 644–666 | CE | ttgggattctccttcatgtcttcTTTTTctcttggaaagaaagt | 1055–1078 | CE | ccctactgctctcagatctctgtgTTTTTctcttggaaagaaagt | |||
| 667–692 | CE | actctagaatcttcttgatctcccttTTTTTctcttggaaagaaagt | 1168–1193 | CE | ggtctgattaccttttgatgaaatagTTTTTctcttggaaagaaagt | |||
| 514–531 | LE | tcccaggtgcctgggtcaTTTTTaggcataggacccgtgtct | 987–1008 | LE | caaaatcctcactttgtgccacTTTTTaggcataggacccgtgtct | |||
| 555–576 | LE | gacccataggacactttcccatTTTTTaggcataggacccgtgtct | 1102–1126 | LE | ggctcaaatagctaaagcatacagtTTTTTaggcataggacccgtgtct | |||
| 577–597 | LE | tccttcacgtgctggtaccacTTTTTaggcataggacccgtgtct | 1127–1145 | LE | cgcaacgcttagggatggaTTTTTaggcataggacccgtgtct | |||
| 598–616 | LE | gcgtctcagctcccaccacTTTTTaggcataggacccgtgtct | 1146–1167 | LE | aatcatagggcacatagaaccgTTTTTaggcataggacccgtgtct | |||
| 693–711 | LE | ggtagagagcgccccagaaTTTTTaggcataggacccgtgtct | 1194–1218 | LE | agcgtaaccaaaaatttctttagaaTTTTTaggcataggacccgtgtct | |||
| 712–736 | LE | aacaattaaatccacagtctcctcaTTTTTaggcataggacccgtgtct | 1219–1244 | LE | tggttgggtatcaataatattcacatTTTTTaggcataggacccgtgtct | |||
| 782–805 | LE | aacttcagttgggatggttgtgtaTTTTTaggcataggacccgtgtct | 1270–1291 | LE | aacttaccgtttattttggcccTTTTTaggcataggacccgtgtct | |||
| 830–851 | LE | ccccaatggtacctttcctcatTTTTTaggcataggacccgtgtct | 1292–1313 | LE | tttttttttttgggttgtagcgTTTTTaggcataggacccgtgtct | |||
| 532–554 | BL | ccatgaagttctccaagaagctt | 1009–1032 | BL | ccatcttcttccggtagtcttcat | |||
| 737–760 | BL | cattttcttgaaggatgtgtggtg | 1079–1101 | BL | caggacatctaggggtccctcta | |||
| 761–781 | BL | gttagccatggggttctcctt | 1245–1269 | BL | cgagttggtgttgtactgaattgtg | |||
| 806–829 | BL | gaagggataaatagtgtggtccat | ||||||
| Sult1b1 (U_92076) | Sult1c2 (AY_005469) | |||||||
| 340–361 | CE | tccgaggtgatggagttttcttTTTTTctcttggaaagaaagt | 689–711 | CE | cttttccattgatgaaggtttcaTTTTTctcttggaaagaaagt | |||
| 509–530 | CE | tccagatattcttcccaggtgcTTTTTctcttggaaagaaagt | 754–780 | CE | agagaatctgatatttgtctcgaatttTTTTTctcttggaaagaaagt | |||
| 655–678 | CE | gctggctatcttcttgatttctttTTTTTctcttggaaagaaagt | 808–828 | CE | tctggatttcatgctttgggtTTTTTctcttggaaagaaagt | |||
| 288–311 | LE | ccaggaacactcagttccaacattTTTTTaggcataggacccgtgtct | 598–620 | LE | catgcagtctttagcatttcgagTTTTTaggcataggacccgtgtct | |||
| 312–339 | LE | caagagttcaacacctgatattcttattTTTTTaggcataggacccgtgtct | 648–666 | LE | gctctgggagcacctggctTTTTTaggcataggacccgtgtct | |||
| 435–454 | LE | catccttgccatttcgagcaTTTTTaggcataggacccgtgtct | 667–688 | LE | aaatactcatcccaggtgcctgTTTTTaggcataggacccgtgtct | |||
| 455–480 | LE | atcaaaatgataataggagacaggaaTTTTTaggcataggacccgtgtct | 712–732 | LE | caaaccaggatccccaacttaTTTTTaggcataggacccgtgtct | |||
| 531–553 | LE | ccacatttccagctaggaatttcTTTTTaggcataggacccgtgtct | 733–753 | LE | cccaccatcctttcacatggtTTTTTaggcataggacccgtgtct | |||
| 554–577 | LE | catgatcaaaccatgaaccataggTTTTTaggcataggacccgtgtct | 829–849 | LE | tgcccataaactgcatcacctTTTTTaggcataggacccgtgtct | |||
| 704–723 | LE | gacgatcctgtccaaggcctTTTTTaggcataggacccgtgtct | 850–873 | LE | ccaccacatcttcatccaaattctTTTTTaggcataggacccgtgtct | |||
| 768–788 | LE | gctgtgggcagatgggtgtaaTTTTTaggcataggacccgtgtct | 874–900 | LE | caaatgatgtctccaggactattttatTTTTTaggcataggacccgtgtct | |||
| 789–810 | LE | ggacttgctgtggtccatcattTTTTTaggcataggacccgtgtct | 945–967 | LE | atggactggtccaggatagatttTTTTTaggcataggacccgtgtct | |||
| 362–386 | BL | tcgattggaagatgtgtctttatta | 621–647 | BL | catcctgtagaagtggtagtaggaaac | |||
| 387–409 | BL | cccagaaggattttgggagtaga | 781–807 | BL | tcctcttcatatcttcatagaagagga | |||
| 410–434 | BL | aggtaaatcatcttgcacttgttct | 901–926 | BL | tgtcataggattctctttcattttct | |||
| 481–508 | BL | caggaagaggattaatactattcatcag | 927–944 | BL | gggggccgtagaacgatt | |||
| 578–600 | BL | cctcttttcccaccaactcttaa | ||||||
| 601–630 | BL | atagtataagtaaagtaaaggatgctcttc | ||||||
| 631–654 | BL | ctttgggttctgtttcaattcttc | ||||||
| 679–703 | BL | cttcatccaaggtcttgtctagaaa | ||||||
| 724–747 | BL | catcatttcaaaggaggtgtgatg | ||||||
| 748–767 | BL | ttgaccagggggttttcctt | ||||||
| Sult1d1 (U_32371) | Sult2a1/2 (L_27121) | |||||||
| 425–447 | CE | actccatttgttatcccaggaatTTTTTctcttggaaagaaagt | 155–175 | CE | agccagttcgttcctgacttgTTTTTctcttggaaagaaagt | |||
| 494–515 | CE | aaggaagcagctgaacaggaagTTTTTctcttggaaagaaagt | 243–262 | CE | tctatccagggtgagcggtcTTTTTctcttggaaagaaagt | |||
| 612–629 | CE | tgccaggctctgggtggaTTTTTctcttggaaagaaagt | 360–377 | CE | gatcgccttggccttggaTTTTTctcttggaaagaaagt | |||
| 630–652 | CE | tttctctaggaactcttcccaggTTTTTctcttggaaagaaagt | 100–126 | LE | ctttcaccacaaacttattacgaatatTTTTTaggcataggacccgtgtct | |||
| 283–306 | LE | taggtggagatcaaaatgtcatcaTTTTTaggcataggacccgtgtct | 176–201 | LE | tctgaatcaagcatacaatctcattcTTTTTaggcataggacccgtgtct | |||
| 329–355 | LE | gtagatcaaatccagtatttcactgacTTTTTaggcataggacccgtgtct | 263–290 | LE | gattattgcagaatatcctatttcagtcTTTTTaggcataggacccgtgtct | |||
| 356–376 | LE | tttctctgcatccccattgttTTTTTaggcataggacccgtgtct | 291–313 | LE | atgagtcgtggtccttccttattTTTTTaggcataggacccgtgtct | |||
| 377–400 | LE | tttgtagattgcatcccttttacaTTTTTaggcataggacccgtgtct | 378–404 | LE | aatatctctcggatttctcatgagataTTTTTaggcataggacccgtgtct | |||
| 401–424 | LE | tataagctccatgaatggtactcgTTTTTaggcataggacccgtgtct | 430–454 | LE | ggattcttcacaaggtttgtgttacTTTTTaggcataggacccgtgtct | |||
| 448–468 | LE | ggcatgttgttcagcatttcaTTTTTaggcataggacccgtgtct | 500–522 | LE | gctcaaaccatgatccgaatagaTTTTTaggcataggacccgtgtct | |||
| 469–493 | LE | gtgtgttttcactattcgaggagacTTTTTaggcataggacccgtgtct | 523–541 | LE | gacagccagccacggacatTTTTTaggcataggacccgtgtct | |||
| 516–538 | LE | gcagtcatttttccagaatgaggTTTTTaggcataggacccgtgtct | 127–154 | BL | gggtaagttaatatcaacaagtcttctt | |||
| 539–561 | LE | ttccgtgccacataaataatcttTTTTTaggcataggacccgtgtct | 202–221 | BL | ccacttcggatctcccttgg | |||
| 674–695 | LE | catgatcataccagggaccaaaTTTTTaggcataggacccgtgtct | 222–242 | BL | ccaaatgggcacagtttggat | |||
| 307–328 | BL | ccaagttgttccagatttggga | 314–333 | BL | ggatgggaagatgggaggtt | |||
| 562–583 | BL | agaaacaaccacatctttggca | 334–359 | BL | actgaagaaagacttggagaagagat | |||
| 584–611 | BL | tttttgccatttgatagaaataatagta | 405–429 | BL | cccagaaaaagtaaccagacacaag | |||
| 653–673 | BL | gctcacttgtccagccatgaa | 455–477 | BL | caaaataagttccgagtgaccct | |||
| 478–499 | BL | acatttccttggaggaaccatt | ||||||
| Sult1e1 (S_78182) | Sult2b1 (AF_026072) | |||||||
| 387–412 | CE | ccttctcttttaattgttttattccaTTTTTctcttggaaagaaagt | 536–558 | CE | taattgcccagcaatcttagaatTTTTTctcttggaaagaaagt | |||
| 413–439 | CE | ggtgagtttttactattctgggagattTTTTTctcttggaaagaaagt | 578–600 | CE | gaggaaattttgaaggaactggtTTTTTctcttggaaagaaagt | |||
| 732–757 | CE | gctttctctccaggaactctattagcTTTTTctcttggaaagaaagt | 688–708 | CE | gtcctgctgcagctcctcataTTTTTctcttggaaagaaagt | |||
| 260–283 | LE | cttcactaatccaggtggtaccagTTTTTaggcataggacccgtgtct | 512–535 | LE | aataatagagggagaccacgacatTTTTTaggcataggacccgtgtct | |||
| 284–313 | LE | catcaccttctttatagatcatatacacaaTTTTTaggcataggacccgtgtct | 559–577 | LE | cgggtgtaccagggtccttTTTTTaggcataggacccgtgtct | |||
| 314–334 | LE | catcctccttgcatttttccaTTTTTaggcataggacccgtgtct | 601–622 | LE | agccaaactgcacttctcctttTTTTTaggcataggacccgtgtct | |||
| 335–361 | LE | ccaaataaggtattctgttaaaaattgTTTTTaggcataggacccgtgtct | 623–643 | LE | ccttgatgtggtcaaaccaggTTTTTaggcataggacccgtgtct | |||
| 362–386 | LE | tttattaggtcttcgtttctgcactTTTTTaggcataggacccgtgtct | 644–662 | LE | ttctgcatccggatccagcTTTTTaggcataggacccgtgtct | |||
| 459–481 | LE | aattcttttcccaaaatgatgctTTTTTaggcataggacccgtgtct | 709–725 | LE | tgcacggagcctcgcagTTTTTaggcataggacccgtgtct | |||
| 582–605 | LE | tgcataaatttctccacaaattcaTTTTTaggcataggacccgtgtct | 726–746 | LE | cccaggaactcacagatgcgtTTTTTaggcataggacccgtgtct | |||
| 627–650 | LE | caagctttcacatgatcataccagTTTTTaggcataggacccgtgtct | 765–783 | LE | caccacagagctcagggccTTTTTaggcataggacccgtgtct | |||
| 758–776 | LE | tccacaagctctgccgaggTTTTTaggcataggacccgtgtct | 803–823 | LE | acatggtattggccttcatggTTTTTaggcataggacccgtgtct | |||
| 440–458 | BL | ggaaggaccttgggtggca | 663–687 | BL | ggtgataaacaggaagttctcttgg | |||
| 482–503 | BL | cggcaaagatagatcatcttgc | 747–764 | BL | tcttcacccagtggccgg | |||
| 504–522 | BL | ggcgacatctttggcgttc | 784–802 | BL | cagcaaaggctgagtgggc | |||
| 523–553 | BL | ttatcattagcaaaaagtagtaataagaaac | ||||||
| 554–581 | BL | gaaaaagatttaggatttggataactag | ||||||
| 606–626 | BL | gaaccatacggaacttgccct | ||||||
| 651–674 | BL | cgtgaattcttactcttttcccac | ||||||
| 675–703 | BL | tcatgtcttcatagaacataaataaaaca | ||||||
| 704–731 | BL | tttacaacttctcttctgatatcctctt | ||||||
| Sult3a1 (AF026075) | Sult5a1 (AF_026074) | |||||||
| 211–231 | CE | aaaggatctgctgggtccagaTTTTTctcttggaaagaaagt | 555–574 | CE | gcctgtgccctcgagaaactTTTTTctcttggaaagaaagt | |||
| 256–278 | CE | ttcgatgttttcagttctgttccTTTTTctcttggaaagaaagt | 719–739 | CE | gatatcctcctcttttggcccTTTTTctcttggaaagaaagt | |||
| 401–425 | CE | catataaagaattttggcttttttgTTTTTctcttggaaagaaagt | 762–786 | CE | actatgttgctctgactcatgaaggTTTTTctcttggaaagaaagt | |||
| 46–73 | LE | ttattgtccataatttgttacctctagtTTTTTaggcataggacccgtgtct | 595–612 | LE | agccaccccttcacgtggTTTTTaggcataggacccgtgtct | |||
| 132–161 | LE | ttcataattttctatattttccactacttcTTTTTaggcataggacccgtgtct | 613–634 | LE | agttaggtccttctgcaggctcTTTTTaggcataggacccgtgtct | |||
| 232–255 | LE | gatgaccctcaaaataaatcaaggTTTTTaggcataggacccgtgtct | 660–678 | LE | aagcgaggttcctggtgcaTTTTTaggcataggacccgtgtct | |||
| 333–351 | LE | tgcgaggtgatggcattttTTTTTaggcataggacccgtgtct | 740–761 | LE | aaaagctgctgtgttccaggatTTTTTaggcataggacccgtgtct | |||
| 426–452 | LE | gatcaaaacatctttaggatttctgtaTTTTTaggcataggacccgtgtct | 809–831 | LE | ccctcgctctggtctatgatctcTTTTTaggcataggacccgtgtct | |||
| 533–554 | LE | aaggcttcctaccacatctccaTTTTTaggcataggacccgtgtct | 855–875 | LE | agtattccctccagttccccaTTTTTaggcataggacccgtgtct | |||
| 74–100 | BL | cctttgaagttaagcaaatattcatct | 876–900 | LE | aacttctcattcagctcaggagtaaTTTTTaggcataggacccgtgtct | |||
| 101–131 | BL | cattttaactaaagttttctgaaaattatag | 923–942 | LE | caaaggccagagtcacccatTTTTTaggcataggacccgtgtct | |||
| 162–186 | BL | caatgaagatgtcatcatctcgaat | 575–594 | BL | tcaaaccaggagccgaagaa | |||
| 187–210 | BL | tggtaccagactttggatatgtga | 635–659 | BL | gctcctcataggtgacaaaaaacaa | |||
| 279–304 | BL | tcaaaaaatggtgctctatctattgt | 679–704 | BL | ggaattcacttaacttgcggatagta | |||
| 305–332 | BL | ggcatagtctaatttgtgaatattgtac | 705–718 | BL | cagggggcgcccca | |||
| 352–375 | BL | aatatggaatgtgggaactgaaga | 787–808 | BL | cttggacagcaggctgtagttg | |||
| 376–400 | BL | tccttgagaccttttggtactaagt | 832–854 | BL | caacacctttcctgaaaaacttg | |||
| 453–478 | BL | atcaaatttgagaaatgaaaatagga | 901–922 | BL | cttggactggtagacagcgttg | |||
| 479–504 | BL | cagtgtctggattttgaaatataagc | ||||||
| 505–532 | BL | tctagaaatgtttgcataaaactttcta | ||||||
| Sult4a1 (AF_059257) | PAPSs1 (U_34883) | |||||||
| 237–252 | CE | gggtcggcaccctggcTTTTTctcttggaaagaaagt | 344–366 | CE | gcgaacgttctcttctctgtcctTTTTTctcttggaaagaaagt | |||
| 373–390 | CE | tcagagggcaggaagcggTTTTTctcttggaaagaaagt | 455–475 | CE | cctcatgaatctgccttgcgtTTTTTctcttggaaagaaagt | |||
| 496–516 | CE | cagaactcctggaaggtgcctTTTTTctcttggaaagaaagt | 578–598 | CE | cgatgccagtgaagccttttaTTTTTctcttggaaagaaagt | |||
| 253–273 | LE | ttcatcaggccgatttcatcaTTTTTaggcataggacccgtgtct | 794–816 | CE | tttattgattttcagggctggtaTTTTTctcttggaaagaaagt | |||
| 274–292 | LE | cggcagctgctcgtcaatgTTTTTaggcataggacccgtgtct | 367–384 | LE | cgccacctcagctatgcgTTTTTaggcataggacccgtgtct | |||
| 293–312 | LE | ggctgtgggtactccagcacTTTTTaggcataggacccgtgtct | 385–405 | LE | gccagcatctgcaaacagcttTTTTTaggcataggacccgtgtct | |||
| 313–332 | LE | ccttgatgatgtccagccccTTTTTaggcataggacccgtgtct | 433–454 | LE | tgttgcgatcctgtgtgtaaggTTTTTaggcataggacccgtgtct | |||
| 411–431 | LE | tgcgagccatgtagatgacctTTTTTaggcataggacccgtgtct | 496–518 | LE | agaggagcatcgacaaaaacttcTTTTTaggcataggacccgtgtct | |||
| 476–495 | LE | cggtagctcatggtccgcagTTTTTaggcataggacccgtgtct | 519–539 | LE | tccctctgctcacagacatgcTTTTTaggcataggacccgtgtct | |||
| 517–538 | LE | tttgtcattcatgaacctccgaTTTTTaggcataggacccgtgtct | 599–622 | LE | cctcgggtttctcatactcagaatTTTTTaggcataggacccgtgtct | |||
| 539–556 | LE | ccaggagccgtagcccagTTTTTaggcataggacccgtgtct | 640–660 | LE | gacgtcacaggaatccgttttTTTTTaggcataggacccgtgtct | |||
| 557–579 | LE | cagaattcctgaacatgctcaaaTTTTTaggcataggacccgtgtct | 661–679 | LE | cctgctggacgcagtcgttTTTTTaggcataggacccgtgtct | |||
| 580–599 | LE | tggcatccattcgatgttccTTTTTaggcataggacccgtgtct | 702–721 | LE | catccacagggacgatgtccTTTTTaggcataggacccgtgtct | |||
| 333–352 | BL | gaggcggggagatgtcagtt | 773–793 | LE | aggcttctgcatcagttttggTTTTTaggcataggacccgtgtct | |||
| 353–372 | BL | tacgggaggtggctcttgat | 817–836 | LE | tgcacccactgcatatccacTTTTTaggcataggacccgtgtct | |||
| 391–410 | BL | tggagtccccattgtggagg | 837–856 | LE | cccaaccttctgccaaaaccTTTTTaggcataggacccgtgtct | |||
| 432–452 | BL | ataccaccaagtccttggggt | 406–432 | BL | cgatataaagctggtgatacacactaa | |||
| 453–475 | BL | cgagcggtggaactgatagtaag | 476–495 | BL | aaagaagggcaagcttgcac | |||
| 540–562 | BL | ccttcttgtagaggcctttgaca | ||||||
| 563–577 | BL | tctcccctgcccgcg | ||||||
| 623–639 | BL | cagcaccagctccgggg | ||||||
| 680–701 | BL | cgttcctgaagaagctccacaa | ||||||
| 722–748 | BL | catatagctctttcacttcataggaag | ||||||
| PAPSs2 (AF_052453) | ||||||||
| 238–256 | CE | tcggaatcctcccctggttTTTTTctcttggaaagaaagt | ||||||
| 412–432 | CE | atattctcttctcggtcccccTTTTTctcttggaaagaaagt | ||||||
| 544–564 | CE | aagaacgggagtcctgctgatTTTTTctcttggaaagaaagt | ||||||
| 612–635 | CE | gggctcgtttgtagagtccttttaTTTTTctcttggaaagaaagt | ||||||
| 257–278 | LE | ctgttagccacacggtacatccTTTTTaggcataggacccgtgtct | ||||||
| 346–367 | LE | cccatccagagagtaacatgggTTTTTaggcataggacccgtgtct | ||||||
| 368–387 | LE | aggccatgacggacattgtcTTTTTaggcataggacccgtgtct | ||||||
| 504–525 | LE | ttctcacgatcctttgcaaaagTTTTTaggcataggacccgtgtct | ||||||
| 526–543 | LE | tcgtggatttttcgggcaTTTTTaggcataggacccgtgtct | ||||||
| 587–611 | LE | cgtctcggctttcacagatatttaaTTTTTaggcataggacccgtgtct | ||||||
| 636–658 | LE | aaaccctttaatctctcctgctcTTTTTaggcataggacccgtgtct | ||||||
| 659–681 | LE | tcatagtcagaatcgatgcctgtTTTTTaggcataggacccgtgtct | ||||||
| 279–299 | BL | ttttcccagcaccagagagac | ||||||
| 300–321 | BL | tccaaagcaaagcttatggttg | ||||||
| 322–345 | BL | atggcgtgagatacaaggtactct | ||||||
| 388–411 | BL | gcagagaatcccaggttcttatta | ||||||
| 433–446 | BL | ccgcgatccggcgg | ||||||
| 447–465 | BL | gcaaagagcctggccacct | ||||||
| 466–480 | BL | accaggccggcgtcg | ||||||
| 481–503 | BL | gagagataaagctggtgatgcaa | ||||||
| 565–586 | BL | aggcgcatctacaaagatctca | ||||||
Targeta . | Functionb . | Sequence . | Target . | Function . | Sequence . | |||
|---|---|---|---|---|---|---|---|---|
| Sult1a1 (L_02331) | Sult1c1 (AF_033653) | |||||||
| 617–643 | CE | atagaagagatagagaacagggtgagtTTTTTctcttggaaagaaagt | 1033–1054 | CE | cggaaggtaatagtgctccctgTTTTTctcttggaaagaaagt | |||
| 644–666 | CE | ttgggattctccttcatgtcttcTTTTTctcttggaaagaaagt | 1055–1078 | CE | ccctactgctctcagatctctgtgTTTTTctcttggaaagaaagt | |||
| 667–692 | CE | actctagaatcttcttgatctcccttTTTTTctcttggaaagaaagt | 1168–1193 | CE | ggtctgattaccttttgatgaaatagTTTTTctcttggaaagaaagt | |||
| 514–531 | LE | tcccaggtgcctgggtcaTTTTTaggcataggacccgtgtct | 987–1008 | LE | caaaatcctcactttgtgccacTTTTTaggcataggacccgtgtct | |||
| 555–576 | LE | gacccataggacactttcccatTTTTTaggcataggacccgtgtct | 1102–1126 | LE | ggctcaaatagctaaagcatacagtTTTTTaggcataggacccgtgtct | |||
| 577–597 | LE | tccttcacgtgctggtaccacTTTTTaggcataggacccgtgtct | 1127–1145 | LE | cgcaacgcttagggatggaTTTTTaggcataggacccgtgtct | |||
| 598–616 | LE | gcgtctcagctcccaccacTTTTTaggcataggacccgtgtct | 1146–1167 | LE | aatcatagggcacatagaaccgTTTTTaggcataggacccgtgtct | |||
| 693–711 | LE | ggtagagagcgccccagaaTTTTTaggcataggacccgtgtct | 1194–1218 | LE | agcgtaaccaaaaatttctttagaaTTTTTaggcataggacccgtgtct | |||
| 712–736 | LE | aacaattaaatccacagtctcctcaTTTTTaggcataggacccgtgtct | 1219–1244 | LE | tggttgggtatcaataatattcacatTTTTTaggcataggacccgtgtct | |||
| 782–805 | LE | aacttcagttgggatggttgtgtaTTTTTaggcataggacccgtgtct | 1270–1291 | LE | aacttaccgtttattttggcccTTTTTaggcataggacccgtgtct | |||
| 830–851 | LE | ccccaatggtacctttcctcatTTTTTaggcataggacccgtgtct | 1292–1313 | LE | tttttttttttgggttgtagcgTTTTTaggcataggacccgtgtct | |||
| 532–554 | BL | ccatgaagttctccaagaagctt | 1009–1032 | BL | ccatcttcttccggtagtcttcat | |||
| 737–760 | BL | cattttcttgaaggatgtgtggtg | 1079–1101 | BL | caggacatctaggggtccctcta | |||
| 761–781 | BL | gttagccatggggttctcctt | 1245–1269 | BL | cgagttggtgttgtactgaattgtg | |||
| 806–829 | BL | gaagggataaatagtgtggtccat | ||||||
| Sult1b1 (U_92076) | Sult1c2 (AY_005469) | |||||||
| 340–361 | CE | tccgaggtgatggagttttcttTTTTTctcttggaaagaaagt | 689–711 | CE | cttttccattgatgaaggtttcaTTTTTctcttggaaagaaagt | |||
| 509–530 | CE | tccagatattcttcccaggtgcTTTTTctcttggaaagaaagt | 754–780 | CE | agagaatctgatatttgtctcgaatttTTTTTctcttggaaagaaagt | |||
| 655–678 | CE | gctggctatcttcttgatttctttTTTTTctcttggaaagaaagt | 808–828 | CE | tctggatttcatgctttgggtTTTTTctcttggaaagaaagt | |||
| 288–311 | LE | ccaggaacactcagttccaacattTTTTTaggcataggacccgtgtct | 598–620 | LE | catgcagtctttagcatttcgagTTTTTaggcataggacccgtgtct | |||
| 312–339 | LE | caagagttcaacacctgatattcttattTTTTTaggcataggacccgtgtct | 648–666 | LE | gctctgggagcacctggctTTTTTaggcataggacccgtgtct | |||
| 435–454 | LE | catccttgccatttcgagcaTTTTTaggcataggacccgtgtct | 667–688 | LE | aaatactcatcccaggtgcctgTTTTTaggcataggacccgtgtct | |||
| 455–480 | LE | atcaaaatgataataggagacaggaaTTTTTaggcataggacccgtgtct | 712–732 | LE | caaaccaggatccccaacttaTTTTTaggcataggacccgtgtct | |||
| 531–553 | LE | ccacatttccagctaggaatttcTTTTTaggcataggacccgtgtct | 733–753 | LE | cccaccatcctttcacatggtTTTTTaggcataggacccgtgtct | |||
| 554–577 | LE | catgatcaaaccatgaaccataggTTTTTaggcataggacccgtgtct | 829–849 | LE | tgcccataaactgcatcacctTTTTTaggcataggacccgtgtct | |||
| 704–723 | LE | gacgatcctgtccaaggcctTTTTTaggcataggacccgtgtct | 850–873 | LE | ccaccacatcttcatccaaattctTTTTTaggcataggacccgtgtct | |||
| 768–788 | LE | gctgtgggcagatgggtgtaaTTTTTaggcataggacccgtgtct | 874–900 | LE | caaatgatgtctccaggactattttatTTTTTaggcataggacccgtgtct | |||
| 789–810 | LE | ggacttgctgtggtccatcattTTTTTaggcataggacccgtgtct | 945–967 | LE | atggactggtccaggatagatttTTTTTaggcataggacccgtgtct | |||
| 362–386 | BL | tcgattggaagatgtgtctttatta | 621–647 | BL | catcctgtagaagtggtagtaggaaac | |||
| 387–409 | BL | cccagaaggattttgggagtaga | 781–807 | BL | tcctcttcatatcttcatagaagagga | |||
| 410–434 | BL | aggtaaatcatcttgcacttgttct | 901–926 | BL | tgtcataggattctctttcattttct | |||
| 481–508 | BL | caggaagaggattaatactattcatcag | 927–944 | BL | gggggccgtagaacgatt | |||
| 578–600 | BL | cctcttttcccaccaactcttaa | ||||||
| 601–630 | BL | atagtataagtaaagtaaaggatgctcttc | ||||||
| 631–654 | BL | ctttgggttctgtttcaattcttc | ||||||
| 679–703 | BL | cttcatccaaggtcttgtctagaaa | ||||||
| 724–747 | BL | catcatttcaaaggaggtgtgatg | ||||||
| 748–767 | BL | ttgaccagggggttttcctt | ||||||
| Sult1d1 (U_32371) | Sult2a1/2 (L_27121) | |||||||
| 425–447 | CE | actccatttgttatcccaggaatTTTTTctcttggaaagaaagt | 155–175 | CE | agccagttcgttcctgacttgTTTTTctcttggaaagaaagt | |||
| 494–515 | CE | aaggaagcagctgaacaggaagTTTTTctcttggaaagaaagt | 243–262 | CE | tctatccagggtgagcggtcTTTTTctcttggaaagaaagt | |||
| 612–629 | CE | tgccaggctctgggtggaTTTTTctcttggaaagaaagt | 360–377 | CE | gatcgccttggccttggaTTTTTctcttggaaagaaagt | |||
| 630–652 | CE | tttctctaggaactcttcccaggTTTTTctcttggaaagaaagt | 100–126 | LE | ctttcaccacaaacttattacgaatatTTTTTaggcataggacccgtgtct | |||
| 283–306 | LE | taggtggagatcaaaatgtcatcaTTTTTaggcataggacccgtgtct | 176–201 | LE | tctgaatcaagcatacaatctcattcTTTTTaggcataggacccgtgtct | |||
| 329–355 | LE | gtagatcaaatccagtatttcactgacTTTTTaggcataggacccgtgtct | 263–290 | LE | gattattgcagaatatcctatttcagtcTTTTTaggcataggacccgtgtct | |||
| 356–376 | LE | tttctctgcatccccattgttTTTTTaggcataggacccgtgtct | 291–313 | LE | atgagtcgtggtccttccttattTTTTTaggcataggacccgtgtct | |||
| 377–400 | LE | tttgtagattgcatcccttttacaTTTTTaggcataggacccgtgtct | 378–404 | LE | aatatctctcggatttctcatgagataTTTTTaggcataggacccgtgtct | |||
| 401–424 | LE | tataagctccatgaatggtactcgTTTTTaggcataggacccgtgtct | 430–454 | LE | ggattcttcacaaggtttgtgttacTTTTTaggcataggacccgtgtct | |||
| 448–468 | LE | ggcatgttgttcagcatttcaTTTTTaggcataggacccgtgtct | 500–522 | LE | gctcaaaccatgatccgaatagaTTTTTaggcataggacccgtgtct | |||
| 469–493 | LE | gtgtgttttcactattcgaggagacTTTTTaggcataggacccgtgtct | 523–541 | LE | gacagccagccacggacatTTTTTaggcataggacccgtgtct | |||
| 516–538 | LE | gcagtcatttttccagaatgaggTTTTTaggcataggacccgtgtct | 127–154 | BL | gggtaagttaatatcaacaagtcttctt | |||
| 539–561 | LE | ttccgtgccacataaataatcttTTTTTaggcataggacccgtgtct | 202–221 | BL | ccacttcggatctcccttgg | |||
| 674–695 | LE | catgatcataccagggaccaaaTTTTTaggcataggacccgtgtct | 222–242 | BL | ccaaatgggcacagtttggat | |||
| 307–328 | BL | ccaagttgttccagatttggga | 314–333 | BL | ggatgggaagatgggaggtt | |||
| 562–583 | BL | agaaacaaccacatctttggca | 334–359 | BL | actgaagaaagacttggagaagagat | |||
| 584–611 | BL | tttttgccatttgatagaaataatagta | 405–429 | BL | cccagaaaaagtaaccagacacaag | |||
| 653–673 | BL | gctcacttgtccagccatgaa | 455–477 | BL | caaaataagttccgagtgaccct | |||
| 478–499 | BL | acatttccttggaggaaccatt | ||||||
| Sult1e1 (S_78182) | Sult2b1 (AF_026072) | |||||||
| 387–412 | CE | ccttctcttttaattgttttattccaTTTTTctcttggaaagaaagt | 536–558 | CE | taattgcccagcaatcttagaatTTTTTctcttggaaagaaagt | |||
| 413–439 | CE | ggtgagtttttactattctgggagattTTTTTctcttggaaagaaagt | 578–600 | CE | gaggaaattttgaaggaactggtTTTTTctcttggaaagaaagt | |||
| 732–757 | CE | gctttctctccaggaactctattagcTTTTTctcttggaaagaaagt | 688–708 | CE | gtcctgctgcagctcctcataTTTTTctcttggaaagaaagt | |||
| 260–283 | LE | cttcactaatccaggtggtaccagTTTTTaggcataggacccgtgtct | 512–535 | LE | aataatagagggagaccacgacatTTTTTaggcataggacccgtgtct | |||
| 284–313 | LE | catcaccttctttatagatcatatacacaaTTTTTaggcataggacccgtgtct | 559–577 | LE | cgggtgtaccagggtccttTTTTTaggcataggacccgtgtct | |||
| 314–334 | LE | catcctccttgcatttttccaTTTTTaggcataggacccgtgtct | 601–622 | LE | agccaaactgcacttctcctttTTTTTaggcataggacccgtgtct | |||
| 335–361 | LE | ccaaataaggtattctgttaaaaattgTTTTTaggcataggacccgtgtct | 623–643 | LE | ccttgatgtggtcaaaccaggTTTTTaggcataggacccgtgtct | |||
| 362–386 | LE | tttattaggtcttcgtttctgcactTTTTTaggcataggacccgtgtct | 644–662 | LE | ttctgcatccggatccagcTTTTTaggcataggacccgtgtct | |||
| 459–481 | LE | aattcttttcccaaaatgatgctTTTTTaggcataggacccgtgtct | 709–725 | LE | tgcacggagcctcgcagTTTTTaggcataggacccgtgtct | |||
| 582–605 | LE | tgcataaatttctccacaaattcaTTTTTaggcataggacccgtgtct | 726–746 | LE | cccaggaactcacagatgcgtTTTTTaggcataggacccgtgtct | |||
| 627–650 | LE | caagctttcacatgatcataccagTTTTTaggcataggacccgtgtct | 765–783 | LE | caccacagagctcagggccTTTTTaggcataggacccgtgtct | |||
| 758–776 | LE | tccacaagctctgccgaggTTTTTaggcataggacccgtgtct | 803–823 | LE | acatggtattggccttcatggTTTTTaggcataggacccgtgtct | |||
| 440–458 | BL | ggaaggaccttgggtggca | 663–687 | BL | ggtgataaacaggaagttctcttgg | |||
| 482–503 | BL | cggcaaagatagatcatcttgc | 747–764 | BL | tcttcacccagtggccgg | |||
| 504–522 | BL | ggcgacatctttggcgttc | 784–802 | BL | cagcaaaggctgagtgggc | |||
| 523–553 | BL | ttatcattagcaaaaagtagtaataagaaac | ||||||
| 554–581 | BL | gaaaaagatttaggatttggataactag | ||||||
| 606–626 | BL | gaaccatacggaacttgccct | ||||||
| 651–674 | BL | cgtgaattcttactcttttcccac | ||||||
| 675–703 | BL | tcatgtcttcatagaacataaataaaaca | ||||||
| 704–731 | BL | tttacaacttctcttctgatatcctctt | ||||||
| Sult3a1 (AF026075) | Sult5a1 (AF_026074) | |||||||
| 211–231 | CE | aaaggatctgctgggtccagaTTTTTctcttggaaagaaagt | 555–574 | CE | gcctgtgccctcgagaaactTTTTTctcttggaaagaaagt | |||
| 256–278 | CE | ttcgatgttttcagttctgttccTTTTTctcttggaaagaaagt | 719–739 | CE | gatatcctcctcttttggcccTTTTTctcttggaaagaaagt | |||
| 401–425 | CE | catataaagaattttggcttttttgTTTTTctcttggaaagaaagt | 762–786 | CE | actatgttgctctgactcatgaaggTTTTTctcttggaaagaaagt | |||
| 46–73 | LE | ttattgtccataatttgttacctctagtTTTTTaggcataggacccgtgtct | 595–612 | LE | agccaccccttcacgtggTTTTTaggcataggacccgtgtct | |||
| 132–161 | LE | ttcataattttctatattttccactacttcTTTTTaggcataggacccgtgtct | 613–634 | LE | agttaggtccttctgcaggctcTTTTTaggcataggacccgtgtct | |||
| 232–255 | LE | gatgaccctcaaaataaatcaaggTTTTTaggcataggacccgtgtct | 660–678 | LE | aagcgaggttcctggtgcaTTTTTaggcataggacccgtgtct | |||
| 333–351 | LE | tgcgaggtgatggcattttTTTTTaggcataggacccgtgtct | 740–761 | LE | aaaagctgctgtgttccaggatTTTTTaggcataggacccgtgtct | |||
| 426–452 | LE | gatcaaaacatctttaggatttctgtaTTTTTaggcataggacccgtgtct | 809–831 | LE | ccctcgctctggtctatgatctcTTTTTaggcataggacccgtgtct | |||
| 533–554 | LE | aaggcttcctaccacatctccaTTTTTaggcataggacccgtgtct | 855–875 | LE | agtattccctccagttccccaTTTTTaggcataggacccgtgtct | |||
| 74–100 | BL | cctttgaagttaagcaaatattcatct | 876–900 | LE | aacttctcattcagctcaggagtaaTTTTTaggcataggacccgtgtct | |||
| 101–131 | BL | cattttaactaaagttttctgaaaattatag | 923–942 | LE | caaaggccagagtcacccatTTTTTaggcataggacccgtgtct | |||
| 162–186 | BL | caatgaagatgtcatcatctcgaat | 575–594 | BL | tcaaaccaggagccgaagaa | |||
| 187–210 | BL | tggtaccagactttggatatgtga | 635–659 | BL | gctcctcataggtgacaaaaaacaa | |||
| 279–304 | BL | tcaaaaaatggtgctctatctattgt | 679–704 | BL | ggaattcacttaacttgcggatagta | |||
| 305–332 | BL | ggcatagtctaatttgtgaatattgtac | 705–718 | BL | cagggggcgcccca | |||
| 352–375 | BL | aatatggaatgtgggaactgaaga | 787–808 | BL | cttggacagcaggctgtagttg | |||
| 376–400 | BL | tccttgagaccttttggtactaagt | 832–854 | BL | caacacctttcctgaaaaacttg | |||
| 453–478 | BL | atcaaatttgagaaatgaaaatagga | 901–922 | BL | cttggactggtagacagcgttg | |||
| 479–504 | BL | cagtgtctggattttgaaatataagc | ||||||
| 505–532 | BL | tctagaaatgtttgcataaaactttcta | ||||||
| Sult4a1 (AF_059257) | PAPSs1 (U_34883) | |||||||
| 237–252 | CE | gggtcggcaccctggcTTTTTctcttggaaagaaagt | 344–366 | CE | gcgaacgttctcttctctgtcctTTTTTctcttggaaagaaagt | |||
| 373–390 | CE | tcagagggcaggaagcggTTTTTctcttggaaagaaagt | 455–475 | CE | cctcatgaatctgccttgcgtTTTTTctcttggaaagaaagt | |||
| 496–516 | CE | cagaactcctggaaggtgcctTTTTTctcttggaaagaaagt | 578–598 | CE | cgatgccagtgaagccttttaTTTTTctcttggaaagaaagt | |||
| 253–273 | LE | ttcatcaggccgatttcatcaTTTTTaggcataggacccgtgtct | 794–816 | CE | tttattgattttcagggctggtaTTTTTctcttggaaagaaagt | |||
| 274–292 | LE | cggcagctgctcgtcaatgTTTTTaggcataggacccgtgtct | 367–384 | LE | cgccacctcagctatgcgTTTTTaggcataggacccgtgtct | |||
| 293–312 | LE | ggctgtgggtactccagcacTTTTTaggcataggacccgtgtct | 385–405 | LE | gccagcatctgcaaacagcttTTTTTaggcataggacccgtgtct | |||
| 313–332 | LE | ccttgatgatgtccagccccTTTTTaggcataggacccgtgtct | 433–454 | LE | tgttgcgatcctgtgtgtaaggTTTTTaggcataggacccgtgtct | |||
| 411–431 | LE | tgcgagccatgtagatgacctTTTTTaggcataggacccgtgtct | 496–518 | LE | agaggagcatcgacaaaaacttcTTTTTaggcataggacccgtgtct | |||
| 476–495 | LE | cggtagctcatggtccgcagTTTTTaggcataggacccgtgtct | 519–539 | LE | tccctctgctcacagacatgcTTTTTaggcataggacccgtgtct | |||
| 517–538 | LE | tttgtcattcatgaacctccgaTTTTTaggcataggacccgtgtct | 599–622 | LE | cctcgggtttctcatactcagaatTTTTTaggcataggacccgtgtct | |||
| 539–556 | LE | ccaggagccgtagcccagTTTTTaggcataggacccgtgtct | 640–660 | LE | gacgtcacaggaatccgttttTTTTTaggcataggacccgtgtct | |||
| 557–579 | LE | cagaattcctgaacatgctcaaaTTTTTaggcataggacccgtgtct | 661–679 | LE | cctgctggacgcagtcgttTTTTTaggcataggacccgtgtct | |||
| 580–599 | LE | tggcatccattcgatgttccTTTTTaggcataggacccgtgtct | 702–721 | LE | catccacagggacgatgtccTTTTTaggcataggacccgtgtct | |||
| 333–352 | BL | gaggcggggagatgtcagtt | 773–793 | LE | aggcttctgcatcagttttggTTTTTaggcataggacccgtgtct | |||
| 353–372 | BL | tacgggaggtggctcttgat | 817–836 | LE | tgcacccactgcatatccacTTTTTaggcataggacccgtgtct | |||
| 391–410 | BL | tggagtccccattgtggagg | 837–856 | LE | cccaaccttctgccaaaaccTTTTTaggcataggacccgtgtct | |||
| 432–452 | BL | ataccaccaagtccttggggt | 406–432 | BL | cgatataaagctggtgatacacactaa | |||
| 453–475 | BL | cgagcggtggaactgatagtaag | 476–495 | BL | aaagaagggcaagcttgcac | |||
| 540–562 | BL | ccttcttgtagaggcctttgaca | ||||||
| 563–577 | BL | tctcccctgcccgcg | ||||||
| 623–639 | BL | cagcaccagctccgggg | ||||||
| 680–701 | BL | cgttcctgaagaagctccacaa | ||||||
| 722–748 | BL | catatagctctttcacttcataggaag | ||||||
| PAPSs2 (AF_052453) | ||||||||
| 238–256 | CE | tcggaatcctcccctggttTTTTTctcttggaaagaaagt | ||||||
| 412–432 | CE | atattctcttctcggtcccccTTTTTctcttggaaagaaagt | ||||||
| 544–564 | CE | aagaacgggagtcctgctgatTTTTTctcttggaaagaaagt | ||||||
| 612–635 | CE | gggctcgtttgtagagtccttttaTTTTTctcttggaaagaaagt | ||||||
| 257–278 | LE | ctgttagccacacggtacatccTTTTTaggcataggacccgtgtct | ||||||
| 346–367 | LE | cccatccagagagtaacatgggTTTTTaggcataggacccgtgtct | ||||||
| 368–387 | LE | aggccatgacggacattgtcTTTTTaggcataggacccgtgtct | ||||||
| 504–525 | LE | ttctcacgatcctttgcaaaagTTTTTaggcataggacccgtgtct | ||||||
| 526–543 | LE | tcgtggatttttcgggcaTTTTTaggcataggacccgtgtct | ||||||
| 587–611 | LE | cgtctcggctttcacagatatttaaTTTTTaggcataggacccgtgtct | ||||||
| 636–658 | LE | aaaccctttaatctctcctgctcTTTTTaggcataggacccgtgtct | ||||||
| 659–681 | LE | tcatagtcagaatcgatgcctgtTTTTTaggcataggacccgtgtct | ||||||
| 279–299 | BL | ttttcccagcaccagagagac | ||||||
| 300–321 | BL | tccaaagcaaagcttatggttg | ||||||
| 322–345 | BL | atggcgtgagatacaaggtactct | ||||||
| 388–411 | BL | gcagagaatcccaggttcttatta | ||||||
| 433–446 | BL | ccgcgatccggcgg | ||||||
| 447–465 | BL | gcaaagagcctggccacct | ||||||
| 466–480 | BL | accaggccggcgtcg | ||||||
| 481–503 | BL | gagagataaagctggtgatgcaa | ||||||
| 565–586 | BL | aggcgcatctacaaagatctca | ||||||
CE, capture extender; LE, label extender; BL, blocker.
Target refers to the sequence of the mRNA transcript as enumerated in the GenBank file.
Function refers to the utility of the oligonucleotide probe in the bDNA assay.
Total RNA (1 μg/μl, 10 μl per well) was added to each well of a 96-well plate containing 50 μl of each diluted probe set. RNA was allowed to hybridize with the probe sets overnight at 53°C. Subsequent hybridization steps were carried out according to the manufacturer's protocol, and luminescence was quantified with a Quantiplex 320 bDNA luminometer interfaced with Quantiplex Data Management software v5.02. Data are presented as relative light units (RLU) per 10 μg of total RNA.
Statistical analysis.
Gender differences in mice were determined using Student's t-test with significance set at p ≤ 0.05. Bars represent mean ± SEM.
RESULTS
Tissue Distribution of Sults
Sult1a1 (phenol Sult) mRNA expression in mice was highest in large intestine, liver, and lung. It was expressed at lower levels in other tissues examined. Sult1a1 mRNA levels in female liver, kidney, and heart were higher than in male mice (Fig. 1). Sult1b1 (dopamine/tyrosine Sult) mRNA was exclusively expressed in the digestive track (stomach, small intestine, and large intestine) (Fig. 1). Sult1c1 (hydroxylamine Sult) mRNA was ubiquitously expressed in all mouse tissues examined except lung. Most of these tissues demonstrate gender bias in Sult1c1 mRNA expression, with higher expression in males than females. The heart, however, had higher levels of Sult1c1 in female than male mice (Fig. 1).
Tissue distribution of Sult1a1, 1b1, 1c1, 1c2, 1d1, and 1e1 in male and female mice. Total RNA was isolated from approximately 8-week-old male and female mice and analyzed by the bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
Sult1c2 mRNA expression in mice was predominant in stomach followed by kidney. Minimal levels of Sult1c2 mRNA were detected in most other tissues. Higher Sult1c2 mRNA expression was observed in female than male kidney, whereas higher expression was observed in male than female stomach (Fig. 1). Sult1d1 mRNA expression was highest in kidney and expressed in all other tissues except heart and brain. Livers obtained from female mice had higher expression of Sult1d1 mRNA than males (Fig. 1). Sult1e1 (estrogen Sult) mRNA expression was highly expressed in testes of male mice and placenta and uterus of female mice (Fig. 1).
Sult2a1/2 (dehydroepiandosterone Sult) mRNA expression was almost exclusively detected in livers obtained from female mice with no expression in male livers. Lower levels of Sult2a1/2 mRNA were detected in brain, gonads, placenta, and uterus (Fig. 2). Sult2b1 mRNA was predominantly expressed in small intestine, followed by lung and stomach of mice (Fig. 2). Sult3a1 mRNA expression was highest in female liver and with low levels in male liver and in all other tissues examined (Fig. 2). Sult4a1 mRNA expression was highest in brain, with fourfold higher levels of expression in females than males (Fig. 3). Sult5a1 mRNA was ubiquitously expressed in all tissues examined except duodenum (Fig. 3).
Tissue distribution of Sult2a1/2 and 2b1 in male and female mice. Total RNA was isolated from approximately 8-week-old male and female mice and analyzed by the bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
Tissue distribution of Sult3a1, 4a1, and 5a1 in male and female mice. Total RNA was isolated from approximately 8-week-old male and female mice and analyzed by the bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
PAPSs1 mRNA was detected in all tissues examined except the liver (Fig. 4). The highest levels of PAPSs1 expression were found in lung, kidney, and uterus. PAPSs2 mRNA expression was highest in duodenum and was not detected in brain, gonads, placenta, or uterus. PAPSs2 mRNA expression in liver and kidney was higher in female than male mice (Fig. 4).
Tissue distribution of for PAPSs1 and PAPSs2 in male and female mice. Total RNA was isolated from approximately 8-week-old male and female mice and analyzed by the bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
Gender differences in tissue distribution.
Sult1a1 mRNA levels in female liver, kidney, and heart were higher than in male mice (Fig. 1). Higher Sult1c1 mRNA expression in males than females were observed in most tissues, except lung (no expression detected), large intestine, brain, and heart. The heart, however, had higher levels of Sult1c1 in female than male mice (Fig. 1). Higher Sult1c2 mRNA was expressed in female than male kidney, whereas higher expression was seen in male than in female stomach (Fig. 1). Sult1d1, 2a1/2, and 3a1 mRNAs in liver were higher in female than male mice (Figs. 1–3). Sult4a1 mRNA expression was higher in female brain, whereas Sult5a1 was higher in male brain (Fig. 3). PAPSs2 mRNA expression was higher in liver and kidney of female than in male mice (Fig. 4).
Ontogeny of Sults
The developmental pattern of mRNA expression was investigated for Sult isozymes in tissues in which they have high expression in mice.
Sult1b1, 1c1, 1c2, 1e1, 4a1, 5a1, and PAPSs1 isozymes have low expression in liver. Therefore, the developmental changes in the hepatic mRNA expression were determined in pooled samples (five animals per sample) to determine whether the mRNA levels were higher at younger ages than in adults. The ontogeny of Sult1c1 and 1c2 demonstrated such a pattern; therefore, they were individually quantified. Sult1b1, 1e1, 4a1, 5a1, and PAPSs1 isozymes were all expressed at undetectable or very low levels in the liver before birth and throughout their postnatal development (data not shown).
Ontogeny in the liver.
Sult1a1 mRNA was expressed at high levels in the liver of mice fetuses 2 days before birth. mRNA levels gradually increased until 22 days of age. Both male and female levels of Sult1a1 mRNA began to decline after 22 days of age but at much faster rates in males than females. Therefore, mRNA levels were higher in livers obtained from female than male mice by 30 or 45 days of age (Fig. 5). Sult1c1 had a unique ontogenic pattern, where hepatic mRNA levels were highest before birth and gradually declined after birth until they reached low levels by 30 days of age (Fig. 5).
Hepatic ontogeny of Sult1a1, 1c1, 1c2, and 1d1 in male and female mice. Total RNA was isolated from livers of − 2-, 0-, 5-, 10-, 22-, 30-, and 45-day-old mice and analyzed by bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
Sult1c2 and 1d1 share similar ontogenic patterns. For these enzymes, mRNA expression in mice liver was very low or undetectable before birth and gradually increased until 10 or 15 days of age. After that, mRNA levels began to decrease in both genders, but at much faster rates in males, with gender differences in hepatic mRNA levels apparent by days 15 or 22 and remained lower in male mice (Fig. 5).
The ontogenic pattern of Sult2a1/2 represents an unusual pattern, where mRNA levels were not detected at birth and then reached a peak at 15 days of age that is much higher than in adults. Livers of adult male mice do not express Sult2a1/2, in contrast to a moderate expression in adult female mice (Fig. 5). Sult3a1 mRNA expression was very low in fetal livers and remained low until 30 days of age, when expression in females markedly increase, whereas it never increased in male mice (Fig. 5).
PAPSs2 mRNA expression gradually increased until 15 days of age and then began to decline, but at a much faster rate in males than females. Therefore, mRNA levels were higher in livers obtained from female than male mice at 30 days of age and older (Fig. 5).
Ontogeny in extrahepatic tissues (kidney, duodenum, and brain).
Sult1b1 mRNA expression gradually increased in the duodenum of mice fetuses from 2 days before birth until 22 days of age, when mRNA levels began to decline in both males and females (Fig. 6). Sult2b1 was expressed at high level in the duodenum of mice fetuses 2 days before birth and gradually increased until 45 days of age in both males and females (Fig. 6).
Ontogeny of Sult1b1, 2b1, and PAPSs2 in duodenum of male and female mice. Total RNA was isolated from livers of − 2-, 0-, 5-, 10-, 22-, 30-, and 45-day-old mice and analyzed by bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
Different ontogenic pattern of PAPSs2 was observed in duodenum than in liver. PAPSs2 was expressed at low levels in duodenum of mice fetuses 2 days before birth and remained low until 10 days of age, when mRNA levels began to increase in both males and females until 45 days of age (Fig. 6).
In kidney, mRNA expression of Sult1c2 gradually increased in mice fetuses from before birth until 22 days of age. However, male levels of Sult1c2 mRNA began to decline after 22 days of age, whereas levels in females remained constant (Fig. 7). Renal mRNA of Sult1d1 continuously increased in mice fetuses 2 days before birth until 45 days of age in both males and females (Fig. 7). The highest expression of renal PAPSs1 was in mice fetuses 2 days before birth and remained unchanged until 15 days of age. After 15 days of age, mRNA levels decreased in both males and females (Fig. 7).
Ontogeny of Sult1c2, 1d1, and PAPSs1 in kidney of male and female mice. Total RNA was isolated from livers of − 2-, 0-, 5-, 10-, 22-, 30-, and 45-day-old mice and analyzed by bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
Sult4a1 in the brain share similar ontogenic pattern as Sult3a1 in the liver. In both enzymes, mRNA expression was very low in fetal livers and brains and remained low until 30 days of age, when expression in females dramatically increase, whereas it never increased in male mice (Fig. 8).
Ontogeny of Sult4a1 in brain of male and female mice. Total RNA was isolated from livers of − 2-, 0-, 5-, 10-, 22-, 30-, and 45-day-old mice and analyzed by bDNA signal amplification assay for mRNA expression. The data are presented as mean RLU ± SEM (n = 5). Asterisk represents a statistically significant difference (p ≤ 0.05) between males and females.
DISCUSSION
Sults catalyze a major pathway of phase-II metabolism of numerous endo- and xenobiotics. Understanding the tissue distribution of Sult isozymes may help identifying their impact on organ-specific pharmacokinetics, pharmacological, and toxicological profiles of numerous substrates. Most studies in the literature concerning the tissue distribution of Sults have been limited to a few tissues and were not quantitative. These previous studies have primarily been performed in rat and human tissues, with limited data available for mice. The mouse is becoming a more common laboratory species because of the availability of the mouse genome sequence as well as transgenic and gene-knockout mice. Therefore, in the present study, we evaluated the relative distribution of the mRNA of Sults in 14 tissues in mice.
Sult1a1 plays essential roles in the metabolism and clearance of various xenobiotics, such as minoxidil and acetaminophen (Hehonah et al., 1999). Sult1a1 has a broad substrate specificity (Chapman et al., 2004; Strott, 2002), yet it preferentially sulfonates simple planar phenols such as 4-nitrophenol (Glatt et al., 2001). In humans, Sult1a1 mRNA is highest in liver of all the Sults (Dooley et al., 2000; Glatt and Meinl, 2004). Sult1a1 is expressed in nearly every human extrahepatic tissue (Glatt and Meinl, 2004), such as kidney, colon, brain, ovary, stomach, small intestine, adrenal gland, and lung (Dooley et al., 2000). In male rats, Sult1a1 mRNA expression is highest in liver followed by lung, adrenal gland, brain, kidney, heart, testes, and intestine (Dunn and Klaassen, 1998). Rat Sult1a1 protein is robustly expressed in livers of males and females from 20 days of age and thereafter (Ozawa et al., 1993). Sult1a1 is also detected in hair follicles in rats, where it may be involved in the sulfation and hair growth–stimulatory activity of minoxidil (Hirshey et al., 1992). Canine Sult1a1 protein is highest in liver and colon, followed by kidney, lung, and small intestine (Tsoi et al., 2002). In mice, Sult1a1 mRNA is detected in kidney, liver, lung, and heart but not in small intestine or spleen (Tamura et al., 1998). The data from the present study demonstrate the expression of Sult1a1 mRNA in all tissues examined, with highest expression in large intestine, liver, and lung in mice. In all species studied, Sult1a1 is expressed in many tissues with highest expression in liver and intestine. The broad tissue expression pattern of Sult1a1 is consistent with a general defense role to efficiently sulfonate a broad range of substrates (Tsoi et al., 2002).
There is a gender difference in expression of Sult1a1 in liver, with higher mRNA levels in livers of female than male mice, which becomes apparent at about 45 days of age (Fig. 5). It was also previously shown that Sult1a1 mRNA expression is higher in female spleen, liver, heart, and kidney than in male mice (Tamura et al., 1999). In rats, Sult1a1 mRNA expression was reported to be sex independent, and Sult1a1 protein was also previously shown to be robustly expressed in livers of males and females at 20 days and 10 weeks of age (Ozawa et al., 1993). However, opposite gender differences in Sult1a1 was reported, where Sult1a1 activity and mRNA expression are about 150% higher in male than female rats (Klaassen et al., 1998; Liu and Klaassen, 1996b). Sult1a1 mRNA levels increase gradually in both male and female rats after birth until puberty and then decline more in female than in male rats (Liu and Klaassen, 1996b). The mechanism underlying this gender difference in rats could neither be attributed to differences in the growth hormone release pattern nor to sex hormones (Klaassen et al., 1998). The difference in gender predominance of hepatic Sult1a1 mRNA expression between mice and rats represents an interesting difference between the two species. Further studies examining the promoter region in both species might identify different response elements responsible for the gender- and species-dependent regulation of Sult1a1.
Sult1b1 catalyzes the sulfo-conjugation of dopamine, tyrosine, and thyroid hormones (Saeki et al., 1998). Dopamine and tyrosine are biosynthetic precursors of catecholamines that function as neurotransmitters in mammals; therefore, Sult1b1 is presumably important for normal functioning of the nervous system (Tamura et al., 1999). The phenolic hydroxyl group of the thyroid hormones (T3 and T4) is subject to sulfo-conjugation by different Sults, including Sult1a1, 1b1, 1c2, and 1e1. However, Sult1b1 has the highest affinity for catalyzing the sulfonation of thyroid hormones (Fujita et al., 1999). The resulting sulfo-conjugates do not bind to nuclear receptors and therefore lack biological activity (Strott, 2002). In humans, Sult1b1 mRNA and protein are reported to be highly expressed in colon, small intestine, spleen, leukocytes, and liver, with highest expression in colon (Wang et al., 1998). Another study in humans reported highest Sult1b1 mRNA expression in stomach, small intestine, colon, liver, ovary, brain and very low in lung (Miki et al., 2002). Also, in humans, the highest level of Sult1b1 protein was found in colon and small intestine, but it was also expressed in liver and leukocytes (Glatt and Meinl, 2004). In rats, both Sult1b1 protein and mRNA are highest in liver followed by kidney but not expressed in any other tissue including intestine (Araki et al., 1997). Previous data from this laboratory, however, reported high expression of Sult1b1 mRNA in small intestine of rats as well as in liver and kidney (Dunn and Klaassen, 1998).
There are gender differences in the mRNA expression of Sult1b1 in livers of rats (Dunn et al., 1999). The ontogenic expression pattern of Sult1b1 in rat liver reveals some gender differences before adulthood (Dunn et al., 1999). In mice, Sult1b1 mRNA was reported to be exclusively expressed in liver (Saeki et al., 1998); however, others have reported expression of Sult1b1 mRNA exclusively in mouse intestine (Tamura et al., 1999). In this current report, Sult1b1 mRNA was expressed mainly in stomach and small and large intestines of mice (Fig. 1). The ontogenic pattern of Sult1b1 was studied in rats (Dunn et al., 1999). Sult1b1 mRNA expression was low in mice livers until 15 days of age, followed by a dramatic increase between 15 and 30 days of age. After that, Sult1b1 mRNA expression in males began to decline and in females remained constant with no gender difference (Dunn et al., 1999). In this current report, there was no gender difference in any of the tissues examined. mRNA levels of Sult1b1 were similar in both male and female in duodenums of mice fetuses and remained similar thereafter (Fig. 6). Therefore, it appears that Sult1b1 is most highly expressed in the intestine of humans, mice, and rats, with no gender difference in expression. This pattern of expression agrees with the suggested role of Sult1b1 of catalyzing the sulfonation of phenolic food constituents in the intestine to detoxify them and accelerate their excretion, as a critical part of the body's defense against potentially harmful compounds (Tamura et al., 1999).
Sult1c substrates include 4-nitrophenol, dopamine, and N-hydroxy-2-acetylaminofluorene (N-OH-2AAF) (Chapman et al., 2004; Hehonah et al., 1999). In humans, Sult1c1 mRNA is detected in kidney, liver, ovary, stomach, and colorectum (Dooley et al., 2000). In rats, Sult1c1 mRNA expression is mainly in liver, with very low or no expression in kidney, spleen, lung, colon, intestine, or brain (Dunn and Klaassen, 1998; Nagata et al., 1993). In a study using mice, Sult1c1 mRNA was only detected in olfactory tissues and was not detected in kidney, intestine, liver, spleen, lung, brain, or heart (Tamura et al., 1998). The data in the present study illustrate an ubiquitous expression of Sult1c1 mRNA in all mouse tissues examined, with highest expression in gonads (Fig. 1).
Sult1c1 mRNA levels were previously reported to be higher in livers of male than female rats, where mRNA expression increased markedly at puberty in male rats but remained low in female rats (Klaassen et al., 1998; Liu and Klaassen, 1996b). Also, Sult1c1 mRNA was detected in livers of both male and female rats at 1 day of age and then decreased to undetectable levels at 7 days of age. Hepatic levels of 1c1 mRNA then increased at more rapid rates in male than female rats and achieved maximum levels at 9 weeks of age with much higher levels in males than females (Nagata et al., 1993). The current data demonstrate in mice a different ontogenic pattern of Sult1c1 mRNA expression, where mRNA levels were highest in livers from mice fetuses 2 days before birth and decreased gradually thereafter (Fig. 5). However, in agreement with other studies in rats, the current study also demonstrates higher Sult1c1 mRNA expression in male than female tissues in mice (Fig. 1). It has been shown that the susceptibility to N-OH-2AAF carcinogenicity in different species parallels the ability of their livers to form the sulfo-conjugate metabolite (DeBaun et al., 1968). Mice, hamsters, and female rats develop tumors more slowly than male rats, when exposed to this carcinogen. Due to this gender difference in Sult1c1 expression, male rats form more sulfo-conjugates of N-OH-2AAF, which causes more tumors in male than female rats (DeBaun et al., 1970). The gender difference in susceptibility to N-OH-2AAF carcinogenicity in mice has not been performed, but it was shown that N-OH-2AAF is metabolized to its sulfo-conjugate and induces tumor formation in male mice (Lai et al., 1987). We expect a gender difference in N-OH-2AAF carcinogenicity in mice due to the predominant expression of Sult1c1 in male over female mice livers.
In adult humans, Sult1c2 mRNA is reported to be only expressed in kidney, stomach, and thyroid gland (Her et al., 1997). However, Sult1c2 mRNA is detected in both kidneys and livers from human fetuses (Her et al., 1997). In rats, Sult1c2 mRNA and protein are highly expressed in kidney, followed by stomach and liver (Xiangrong et al., 2000). In rabbits, Sult1c2 protein is highly expressed in large intestine, kidney, and stomach; moderately expressed in duodenum; and very weakly expressed in ileum, jejunum, liver, and lung (Hehonah et al., 1999). In mice, Sult1c2 mRNA expression was reported to be limited to kidney and stomach (Sugimura et al., 2002). Sult1c2 mRNA was not expressed in brain, heart, liver, lung, skeletal muscle, skin, small intestine, spleen, testis, or thymus. However, large intestine was not examined (Sugimura et al., 2002). Sult1c2 protein was demonstrated to be expressed only in stomach and kidney of mice (Xiangrong et al., 2000). In agreement with previous reports, the data in this article show predominant expression of Sult1c2 mRNA in stomach and kidney of mice (Fig. 1). Therefore, it can be concluded that kidney and stomach are the major organs of Sult1c2 expression in humans, rabbits, rats, and mice.
Sult1c2 mRNA levels were undetectable in livers obtained from fetuses 2 days before birth, but they gradually increased after birth to reach a maximum at 15 days and then decreased much more rapidly in male than female mice, resulting in higher mRNA levels in females 15 days after birth and remained higher thereafter (Fig. 5). Renal Sult1c2 mRNA was expressed at high levels in fetuses 2 days before birth and remained constant after birth until 10 days, when mRNA levels began to increase. However, 22 days after birth, mRNA levels began to decline in male kidneys, whereas female levels remained constant (Fig. 7). Sult1d1 has high substrate specificity for catecholamines, such as serotonin and dopamine, and 4-nitrophenol (Nagata and Yamazoe, 2000). 3,4-Dihydroxyphenylacetic acid is a selective substrate for Sult1d1 (Eisenhofer et al., 1999; Shimada et al., 2004). In dogs, the highest expression of Sult1d1 protein was detected in colon and kidney, followed by small intestine, liver, and lung (Tsoi et al., 2001). Livers from female dogs expressed higher levels of Sult1d1 protein than males (Tsoi et al., 2001). In mice, Sult1d1 mRNA was predominantly expressed in kidney, followed by uterus and liver (Sakakibara et al., 1998). Sult1d1 protein was detected in liver, lung, uterus, and kidney but not in brain of mice, with the highest levels in kidney (only tissues studied) (Shimada et al., 2004). Hepatic Sult1d1 protein was higher in livers of male than female mice (Shimada et al., 2004). Immunohistochemical studies detected Sult1d1 in proximal and distal tubules, as well as in collecting ducts, the same localization of the organic cation transporter (Oct2). Because catecholamines, such as dopamine and norepinephrine, are excreted in urine as sulfates in human, rats, and mice, it was suggested that Oct2 transports dopamine into proximal tubules by Oct2, where it is then sulfated by Sult1d1 (Shimada et al., 2004), which is abundantly expressed in kidney. The current data also demonstrate the highest expression of Sult1d1 in kidneys of mice, but it was also expressed in all other tissues, except heart and brain (Fig. 1). The hepatic mRNA expression of Sult1d1 was predominant in females, and this gender difference did not appear until 30 days of age (Fig. 5). However, there was no gender difference in renal mRNA expression at any age (Fig. 7). Sult1d1 appears to be highest expressed in kidney and intestine of humans, dogs, rats, and mice, and female predominant in liver.
Sult1e1 sulfonates a variety of estrogens, including the drug diethinyl estradiol (Falany et al., 1995). Estrogen plays important roles in both female and male reproductive tissues, bone, liver, and central nervous system (Miki et al., 2002). The inactive estrogen sulfate is a predominant form of estrogen in blood, urine, and milk (Miki et al., 2002). Sult1e1 has the highest affinity for estrogen and is therefore termed estrogen Sult. Sult1e1 and the sulfatase enzymes are responsible for determining the ratio of the active free estrogen to the inactive estrogen sulfate in local tissue environments. Sult1e1 activity in normal breast cells is much higher than that in breast cancer cells. Sult1e1 in normal breast tissues serves an important role in inhibiting excessive estrogenic action and therefore protecting the tissue from cancer (Miki et al., 2002).
The tissue distribution of Sult1e1 appears to be species specific. For example, Sult1e1 mRNA could be detected only in livers of young mature male rats but not in testes (placenta was not examined) (Dunn and Klaassen, 1998; Klaassen et al., 1998; Liu and Klaassen, 1996b), whereas in guinea pigs, Sult1e1 mRNA expression and activity were predominant in the adrenal gland, and very low levels were detected in testes, ovary, liver, placenta, and uterus (Hobkirk and Glasier, 1992; Oeda et al., 1992). In C57BL/KsJ mice, Sult1e1 mRNA expression was exclusive to testis (placenta was not examined) (Song et al., 1995). In CD-1 mice, Sult1e1 activity was predominant in testis, and lower levels were detected in placenta and uterus (Hobkirk and Glasier, 1992). In humans, Sult1e1 mRNA was detected in liver, small intestine, adrenal, and leukocytes, but not in testes or placenta (Her et al., 1996). Another study, however, detected the expression of Sult1e1 mRNA in 24 human tissues, including testes, placenta, liver, kidney, lung, adrenal, and intestine (Miki et al., 2002). Sult1e1 protein was also detected in liver, jejunum, and endometrium in humans (Falany et al., 1995; Forbes-Bamforth and Coughtrie, 1994). Sult1e1 activity was found in both male and female human reproductive tissues, liver, kidney, brain, and adrenal cortex (Hobkirk, 1985). In human fetuses, however, Sult1e1 mRNA was highly expressed in kidney, liver, lung, and intestine (testes were not examined) (Her et al., 1996; Miki et al., 2002). The data in the present study demonstrate that Sult1e1 mRNA expression is predominant in testes and placenta of mice (Fig. 1). Despite the apparent differences in the tissue distribution pattern of Sult1e1 among species, it can be concluded that Sult1e1 is mainly expressed in male and female sex organs as well as placenta.
Sult2a1 has a broad substrate specificity, including DHEA, bile acids, pregnenolone, testosterone, estradiol, and estrone. In humans, Sult2a1 mRNA is exclusively expressed in liver and intestine (Otterness et al., 1995). However, human data from another study reported high expression of Sult2a1 mRNA in adrenal, colon, ovary, prostate, small intestine, stomach, and liver and lower expression in kidney, brain, placenta, spleen, and bone marrow (Dooley et al., 2000; Javitt et al., 2001). In human fetuses, Sult2a1 protein was detected by immunohistochemistry in adrenal cortex, liver, testis, and intestine; weakly detected in kidney; and was not detected in lung, brain, heart, stomach, skeletal muscle, pancreas, and spleen (Parker et al., 1994). The fetal adrenal gland produces large amounts of DHEA sulfate to support placental estrogen biosynthesis, which is reflected by the high levels of Sult2a1 expression in fetal adrenal gland (Coughtrie, 2002).
In female rats, Sult2a1 mRNA expression was exclusive to liver and adrenal (Dunn and Klaassen, 1998). Hepatic Sult2a1 mRNA is female predominant in rats (Chatterjee et al., 1987; Dunn and Klaassen, 1998; Runge-Morris and Wilusz, 1991). Treatment with dihydrotestosterone downregulates Sult2a1 expression in rats (Demyan et al., 1992). Also, hepatic Sult2a1 is upregulated in testicular feminized male mice (Chatterjee et al., 1987; Demyan et al., 1992). These mice lack a functional androgen receptor and therefore are insensitive to androgens due to a frameshift mutation, which results in a premature stop codon (He et al., 1994).
Sult2a1 is a senescence enzyme as mRNA levels are very low in livers obtained from male rats at 6, 12, and 18 months of age; however, mRNA levels dramatically increase at 24 and 27 months of age (Echchgadda et al., 2004). Another study reported the presence of Sult2a1 mRNA expression in livers obtained from 1- to 26-month-old male rats and the absence of expression in those obtained from 3-month-old rats (Demyan et al., 1992).
Similar age- and sex-dependent expression patterns of Sult2a1 have been reported for mice. Sult2a1 mRNA expression is high in livers obtained from female mice, whereas undetectable in male mice (Wu et al., 2001). Hepatic activity of Sult2a1 in female mice is 70-fold higher than in male mice (Borthwick et al., 1995). In whole mouse embryos, Sult2a1 mRNA is undetectable and does not appear until day E19 (Shimizu et al., 2003).
The present data demonstrate similar patterns of tissue distribution and gender- and age-dependent expression of Sult2a1/2 in mice as previously reported. Sult2a1/2 mRNA expression was almost exclusive to livers from female mice (Fig. 2). Sult2a1/2 mRNA was not detected in livers obtained from mice fetuses 2 days before birth but gradually increased after birth in both genders until it reached a maximum 15 days after birth. After that, mRNA levels in the female livers decreased, whereas it completely disappeared in males (Fig. 5). A very similar ontogenic pattern in hepatic Sult2a1 mRNA expression was previously reported in rats (Klaassen et al., 1998; Liu and Klaassen, 1996a). However, the decrease in Sult2a1 mRNA expression in male rats was not as marked and therefore remains detectable in livers of adult male rats (Klaassen et al., 1998). Therefore, it can be concluded that Sult2a1 is a senescence enzyme in both rats and mice, where hepatic Sult2a1 expression dramatically increases during adolescence and starts declining with rising androgen levels during puberty. However, Sult2a1 expression increases again later in life with the diminishment of androgen levels that accompanies senescence.
As a member of the Sult2 family, Sult2b substrates overlap with some Sult2a substrates. Sult2b1 substrates include cholesterol and pregnenolone. Sult2b1 does not sulfonate bile acids, DHEA, or estradiol, whereas Sult2a1 does not sulfonate cholesterol (Strott, 2002). In humans, Sult2b1 mRNA was detected in small intestine, placenta, colon, spleen, ovary, uterus, and prostate and was not detected in any fetal tissue (Geese and Raftogianis, 2001). Additionally, data from human tissues indicate that Sult2b1 mRNA is most prominent in small intestine, colon, stomach, lung, kidney, placenta, prostate, skin, thymus, and thyroid (Javitt et al., 2001). In mice, Sult2b1 mRNA is highly expressed in small intestine and skin, followed by skeletal muscle, prostate, lung, and spleen. Tissues including brain, heart, kidney, liver, stomach, testes, and uterus have barely detectable levels of expression (Shimizu et al., 2003). Another study in mice reported highest Sult2b1 mRNA expression in small intestine and epididymis, followed by uterus (Sakakibara et al., 1998). The data in this article demonstrate predominant expression of Sult2b1 mRNA in mice small intestine, followed by stomach and lung (Fig. 2). Furthermore, Sult2b1 is expressed at high levels in the duodenum of fetuses and gradually increased after birth with no gender difference at any age (Fig. 6). Therefore, small intestine appears to be the tissue of highest Sult2b1 expression in humans, rats, and mice.
There are no previous reports of Sult3a1 tissue distribution in rats, humans, or mice. The current data represent the first report of the tissue distribution of this Sult in mice. Sult3a1 was originally isolated and characterized from rabbit liver. Sult3a1 was the only Sult isozyme that predominantly catalyzes N-sulfonation, rather than O-sulfonation. Phenyl tetrahydropyridine, a potent neurotoxin that induces neurodegenerative diseases, was identified as the best substrate for Sult3a1 (Yoshinari et al., 1998). Sult3a1 mRNA expression is predominant in livers from female mice and is expressed at very low levels in all other tissues examined (Fig. 3). Sult3a1 mRNA levels were very low in livers obtained from fetuses 2 days before birth and remained low until day 30, when levels of Sult3a1 markedly increased in female mice and male levels remained low (Fig. 5).
In humans and rats, Sult4a1 mRNA is exclusively expressed in brain (cerebellum, hypothalamus, cerebrum, and medulla), with higher levels in male than female rats (Falany et al., 2000; Sakakibara et al., 2002). In mice, Sult4a1 mRNA and protein are expressed exclusively in brain and not detected in liver, lung, kidney, uterus, intestine, adrenal, thymus, spleen, testes, stomach, ovary, or heart (Sakakibara et al., 2002). In agreement with the previous reports, the data in this study demonstrate the exclusive expression of Sult4a1 mRNA in brain, with much higher levels in female than male mice (Fig. 3). The selective expression of Sult4a1 in brain of three species suggests an important role for this protein in the central nervous system, which yet has to be investigated by understanding the substrate specificity of this Sult isozyme.
The developmental changes of Sult4a1 expression in the brain are studied here for the first time. Sult4a1 mRNA levels were very low in brains obtained from fetuses 2 days before birth and remained low until day 30, when Sult3a1 markedly increased in female mice and male levels remained low (Fig. 8).
There are no previous reports of Sult5a1 tissue distribution in rats, humans, or mice. The current data represent the first report of the tissue distribution of Sult5a1 in mice. Sult5a1 mRNA was ubiquitously expressed in all tissues examined except stomach (Fig. 3).
PAPS is the universal donor of the sulfonate group, which is used by Sults to catalyze the sulfo-conjugation of substrates. PAPS is formed from dietary inorganic sulfate and ATP by the action of two enzymes, ATP sulfurylase and APS kinase. In mammals, the two enzyme activities are contained within one bifunctional protein, termed PAPSs. Two PAPSs isoenzymes have been cloned from humans and mice, namely, PAPSs1 and PAPSs2. The two isoenzymes differ in their tissue distribution and catalytic activity. PAPSs1 localizes to the nucleus, whereas PAPSs2 localizes to the cytoplasm, except for tissues where PAPSs1 is also expressed (Besset et al., 2000). PAPSs2 has greater catalytic efficiency for PAPS synthesis than PAPSs1 (Fuda et al., 2002). A nonsense mutation in the PAPSs2 gene causes a recessive dwarfing syndrome in mice (bradychymorphism) and humans (spondyloepimetaphyseal dysplasia) (ul Haque et al., 1998). This cartilage-specific defect occurs in spite of coexpression of PAPSs1 in the cartilaginous tissues (Fuda et al., 2002). Also, there are other genetic disorders caused by mutations in transporters involved in the cellular uptake of inorganic sulfate, causing undersulfation of cartilaginous proteoglycans (Strott, 2002).
In humans, PAPSs1 mRNA was detected in 15 tissues but not in liver (Fuda et al., 2002; Girard et al., 1998). PAPSs2, however, was detected in liver as well as all other tissues, except for brain (Fuda et al., 2002). In another study, PAPSs1 mRNA was detected in 22 different human tissues but not in liver and skeletal muscle. PAPSs2, however, was highest in liver and detected in most tissues (Xu et al., 2000). The current data obtained from mice are similar to human data. PAPSs1 mRNA was detected in all mice tissues examined except liver (Fig. 4). PAPSs2, however, was abundantly expressed in mouse gastrointestinal tract, kidney, and liver but was not expressed in heart, brain, gonad, placenta, and uterus (Fig. 4). Therefore, it appears that PAPSs1 is not expressed in liver; however, PAPSs2 is expressed in livers of both humans and mice.
The mRNA expression of PAPSs1 was high in duodenum before birth and remained the same until 22 days of age, when mRNA levels began to decline (Fig. 6). Hepatic and renal PAPSs2 expression was female predominant in mice. PAPSs2 mRNA was expressed in livers obtained from mice 2 days before birth and remained essentially the same with age. The gender difference in hepatic PAPSs2 mRNA expression does not appear until 30 days of age (Fig. 5). However, in duodenum, PAPSs2 mRNA was expressed at very low levels until 10 days after birth, when mRNA levels began to increase equally in both males and females (Fig. 6).
In summary, the present data demonstrate the tissue distribution of 11 Sults and 2 PAPSs mRNAs in 14 tissues of mice. Developmental changes in hepatic mRNA expression were also determined. Sult2a1/2 and 3a1 expression are highest in liver; Sult1b1, 2b1, and PAPSs2 in small intestine; Sult1a1 in large intestine; Sult1c2 in stomach; Sult1d1 in kidney; Sult1e1 in placenta; and Sult4a1 in brain; whereas Sult1c1, 5a1, and PAPSs1 are ubiquitously expressed. Sult isozymes demonstrate different patterns of ontogenic expression. Sult1a1, 1c2, 1d1, 2a1/2, and PAPSs2 hepatic expression gradually increase until about 3 weeks after birth and decline thereafter. Sult1c1 expression is highest before birth and decline thereafter. However, Sult3a1 mRNA expression was very low in fetal livers and remained low until 30 days of age, when expression in females markedly increased but not in males.
We thank David Buckley, Peizhen Song, Xiaohong Lei, and Drs. Chuan Chen and Hong Lu for technical assistance. This work was supported by National Institutes of Health grants ES-09649 and ES-09716.
References
Araki, Y., Sakakibara, Y., Boggaram, V., Katafuchi, J., Suiko, M., Nakajima, H., and Liu, M. C. (
Besset, S., Vincourt, J. B., Amalric, F., and Girard, J. P. (
Borthwick, E. B., Burchell, A., and Coughtrie, M. W. (
Buhl, A. E., Waldon, D. J., Baker, C. A., and Johnson, G. A. (
Chapman, E., Best, M. D., Hanson, S. R., and Wong, C. H. (
Chatterjee, B., Majumdar, D., Ozbilen, O., Murty, C. V., and Roy, A. K. (
Coughtrie, M. W. (
DeBaun, J. R., Miller, E. C., and Miller, J. A. (
DeBaun, J. R., Rowley, J. Y., Miller, E. C., and Miller, J. A. (
Demyan, W. F., Song, C. S., Kim, D. S., Her, S., Gallwitz, W., Rao, T. R., Slomczynska, M., Chatterjee, B., and Roy, A. K. (
Dooley, T. P., Haldeman-Cahill, R., Joiner, J., and Wilborn, T. W. (
Dunn, R. T., II, Gleason, B. A., Hartley, D. P., and Klaassen, C. D. (
Dunn, R. T., II, and Klaassen, C. D. (
Echchgadda, I., Song, C. S., Oh, T. S., Cho, S. H., Rivera, O. J., and Chatterjee, B. (
Eisenhofer, G., Coughtrie, M. W., and Goldstein, D. S. (
Falany, C. N., Krasnykh, V., and Falany, J. L. (
Falany, C. N., Xie, X., Wang, J., Ferrer, J., and Falany, J. L. (
Forbes-Bamforth, K. J., and Coughtrie, M. W. (
Fuda, H., Shimizu, C., Lee, Y. C., Akita, H., and Strott, C. A. (
Fujita, K., Nagata, K., Yamazaki, T., Watanabe, E., Shimada, M., and Yamazoe, Y. (
Geese, W. J., and Raftogianis, R. B. (
Girard, J. P., Baekkevold, E. S., and Amalric, F. (
Glatt, H. (
Glatt, H., Bartsch, I., Christoph, S., Coughtrie, M. W., Falany, C. N., Hagen, M., Landsiedel, R., Pabel, U., Phillips, D. H., Seidel, A., et al. (
Glatt, H., Boeing, H., Engelke, C. E., Ma, L., Kuhlow, A., Pabel, U., Pomplun, D., Teubner, W., and Meinl, W. (
Glatt, H., and Meinl, W. (
He, W. W., Lindzey, J. K., Prescott, J. L., Kumar, M. V., and Tindall, D. J. (
Hehonah, N., Zhu, X., Brix, L., Bolton-Grob, R., Barnett, A., Windmill, K., and McManus, M. (
Her, C., Kaur, G. P., Athwal, R. S., and Weinshilboum, R. M. (
Her, C., Szumlanski, C., Aksoy, I. A., and Weinshilboum, R. M. (
Hirshey, S. J., Dooley, T. P., Reardon, I. M., Heinrikson, R. L., and Falany, C. N. (
Hobkirk, R. (
Hobkirk, R., and Glasier, M. A. (
Javitt, N. B., Lee, Y. C., Shimizu, C., Fuda, H., and Strott, C. A. (
Klaassen, C. D., Liu, L., and Dunn, R. T., II. (
Lai, C. C., Miller, E. C., Miller, J. A., and Liem, A. (
Liu, L., and Klaassen, C. D. (
Liu, L., and Klaassen, C. D. (
Miki, Y., Nakata, T., Suzuki, T., Darnel, A. D., Moriya, T., Kaneko, C., Hidaka, K., Shiotsu, Y., Kusaka, H., and Sasano, H. (
Nagata, K., Ozawa, S., Miyata, M., Shimada, M., Gong, D. W., Yamazoe, Y., and Kato, R. (
Nagata, K., and Yamazoe, Y. (
Niehrs, C., Beisswanger, R., and Huttner, W. B. (
Oeda, T., Lee, Y. C., Driscoll, W. J., Chen, H. C., and Strott, C. A. (
Otterness, D. M., Her, C., Aksoy, S., Kimura, S., Wieben, E. D., and Weinshilboum, R. M. (
Ozawa, S., Nagata, K., Gong, D. W., Yamazoe, Y., and Kato, R. (
Parker, C. R., Jr., Falany, C. N., Stockard, C. R., Stankovic, A. K., and Grizzle, W. E. (
Runge-Morris, M., and Wilusz, J. (
Saeki, Y., Sakakibara, Y., Araki, Y., Yanagisawa, K., Suiko, M., Nakajima, H., and Liu, M. C. (
Sakakibara, Y., Suiko, M., Pai, T. G., Nakayama, T., Takami, Y., Katafuchi, J., and Liu, M. C. (
Sakakibara, Y., Yanagisawa, K., Takami, Y., Nakayama, T., Suiko, M., and Liu, M. C. (
Shimada, M., Terazawa, R., Kamiyama, Y., Honma, W., Nagata, K., and Yamazoe, Y. (
Shimizu, C., Fuda, H., Yanai, H., and Strott, C. A. (
Song, W. C., Moore, R., McLachlan, J. A., and Negishi, M. (
Sugimura, K., Tanaka, T., Tanaka, Y., Takano, H., Kanagawa, K., Sakamoto, N., Ikemoto, S., Kawashima, H., and Nakatani, T. (
Tamura, H., Miyawaki, A., Yoneshima, H., Mikoshiba, K., and Matsui, M. (
Tamura, H. O., Harada, Y., Miyawaki, A., Mikoshiba, K., and Matsui, M. (
Tsoi, C., Falany, C. N., Morgenstern, R., and Swedmark, S. (
Tsoi, C., Morgenstern, R., and Swedmark, S. (
ul Haque, M. F., King, L. M., Krakow, D., Cantor, R. M., Rusiniak, M. E., Swank, R. T., Superti-Furga, A., Haque, S., Abbas, H., Ahmad, W., et al. (
Wang, J., Falany, J. L., and Falany, C. N. (
Wu, W., Kocarek, T. A., and Runge-Morris, M. (
Xiangrong, L., Johnk, C., Hartmann, D., Schestag, F., Kromer, W., and Gieselmann, V. (
Xu, Z. H., Otterness, D. M., Freimuth, R. R., Carlini, E. J., Wood, T. C., Mitchell, S., Moon, E., Kim, U. J., Xu, J. P., Siciliano, M. J., et al. (
Yamazoe, Y., Nagata, K., Ozawa, S., and Kato, R. (



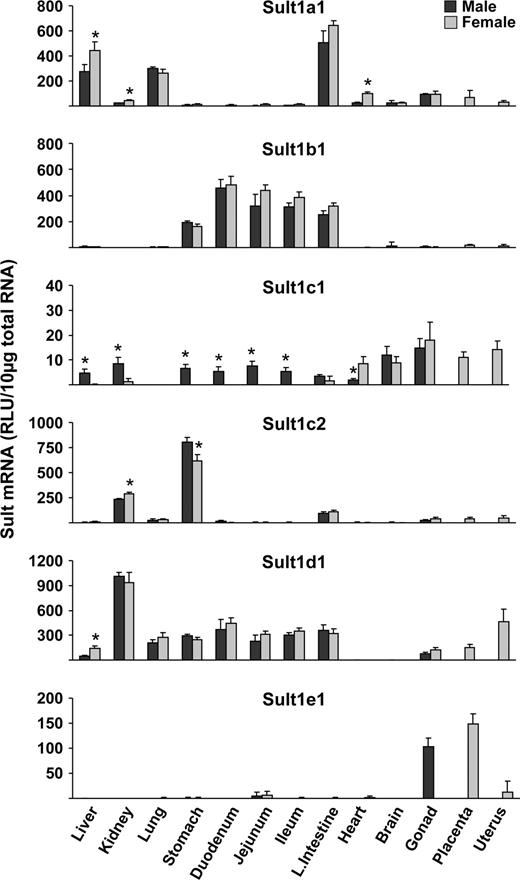
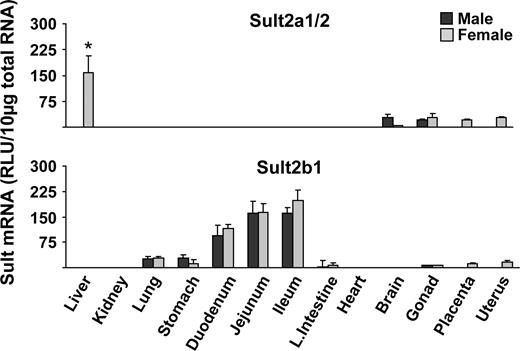
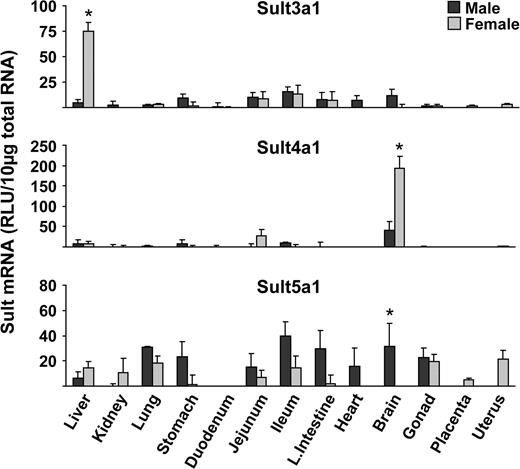
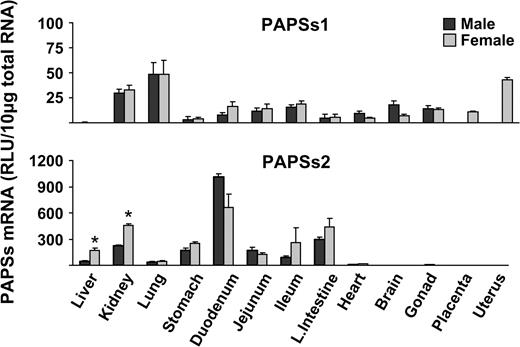
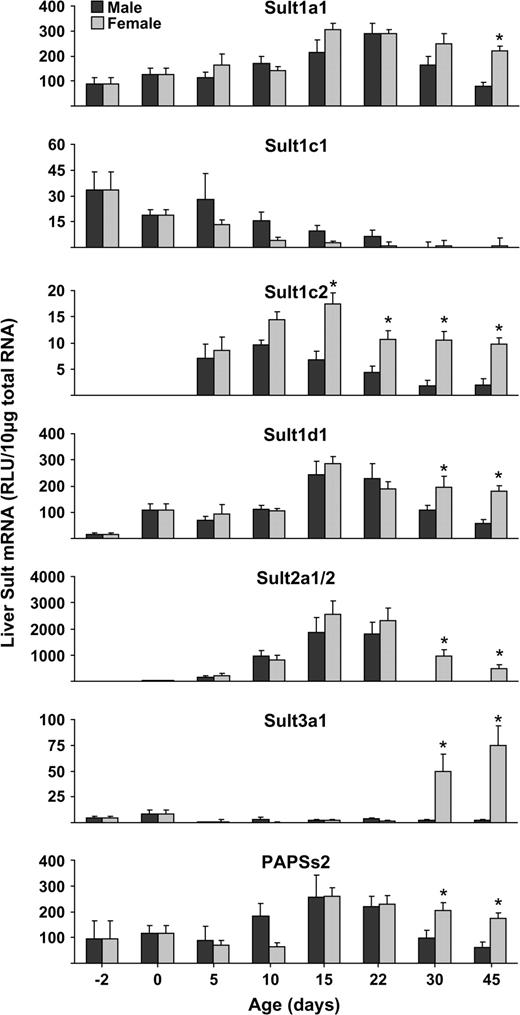
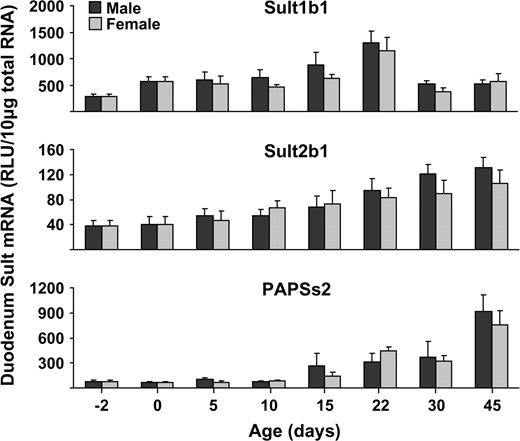
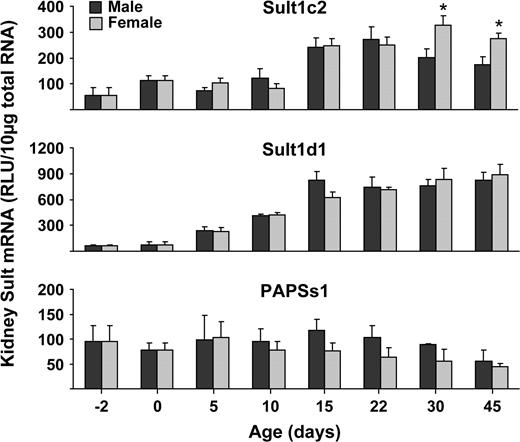
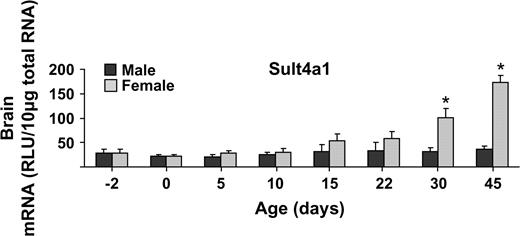

Comments