Abstract
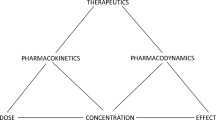
Recently, drug transporters have emerged as significant modifiers of a patient’s pharmacokinetics. In cases where the functioning of drug transporters is altered, such as by drug-drug interactions, by genetic polymorphisms, or as evidenced in knockout animals, the resulting change in volume of distribution can lead to a significant change in drug effect or likelihood of toxicity, as well as a change in half life independent of a change in clearance. Here, we review pharmacokinetic interactions at the transporter level that have been investigated in animals and humans and reported in literature, with a focus on the changes in distribution volume. We pay particular attention to the differing effects of changes in transporter function on the three measures of volume. Further, trends are discussed as they may be used to predict volume changes given the function of a transporter and the primary location of the interaction. Because the liver and kidneys express the greatest level and variety of transporters, we denote these organs as the primary location of transporter-based interactions. We conclude that the liver is a larger contributor to distribution volume than the kidneys, in consideration of both uptake and efflux transporters. Further, while altered distribution due to secondary interactions at tissues other than the liver and kidneys may have a pharmacodynamic effect, these interactions, at least at the blood-brain barrier, do not appear to significantly influence overall distribution volume. The analysis provides a framework for understanding potential pharmacokinetic interactions rooted in drug transporters as they modify drug distribution.

Similar content being viewed by others
References
Øie S. Drug distribution and binding. J Clin Pharmacol. 1986;26:583–6.
Jette L, Beaulieu E, Leclerc JM, Beliveau R. Cyclosporin A treatment induces overexpression of P-glycoprotein in the kidney and other tissues. Am J Physiol. 1996;270:F756–F65.
Brady JM, Cherrington NJ, Hartley DP, Buist SC, Li N, Klaassen CD. Tissue distribution and chemical induction of multiple drug resistance genes in rats. Drug Metab Dispos. 2002;30:838–44.
Cherrington NJ, Hartley DP, Li N, Johnson DR, Klaassen CD. Organ distribution of multidrug resistance proteins 1, 2, and 3 (Mrp1, 2, and 3) mRNA and hepatic induction of Mrp3 by constitutive androstane receptor activators in rats. J Pharmacol Exp Ther. 2002;300:97–104.
Nakashima E, Benet LZ. General treatment of mean residence time, clearance, and volume parameters in linear mammillary models with elimination from any compartment. J Pharmacokinet Biopharm. 1988;16:475–92.
Yates JW, Arundel PA. Oral and IV dosing: a method to determine the compartment of drug elimination for two-compartment models. J Pharm Sci. 2008;97:2036–40.
Yates JW, Arundel PA. On the volume of distribution at steady state and its relationship with two-compartmental models. J Pharm Sci. 2008;97:111–22.
Kerr DJ, Graham J, Cummings J, Morrison JG, Thompson GG, Brodie MJ, Kaye SB. The effect of verapamil on the pharmacokinetics of adriamycin. Cancer Chemother Pharmacol. 1986;18:239–42.
Tavoloni N, Guarino AM. Biliary and urinary excretion of adriamycin in anesthetized rats. Pharmacology. 1980;20:256–67.
Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204.
Pasanen MK, Fredrikson H, Neuvonen PJ, Niemi M. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82:726–33.
Brown G, Zemcov SJ, Clarke AM. Effect of probenecid on cefazolin serum concentrations. J Antimicrob Chemother. 1993;31:1009–11.
Shitara Y, Sato H, Sugiyama Y. Evaluation of drug-drug interaction in the hepatobiliary and renal transport of drugs. Annu Rev Pharmacol Toxicol. 2005;45:689–723.
Sakurai Y, Motohashi H, Ogasawara K, Terada T, Masuda S, Katsura T, Mori N, Matsuura M, Doi T, Fukatsu A, Inui K. Pharmacokinetic significance of renal OAT3 (SLC22A8) for anionic drug elimination in patients with mesangial proliferative glomerulonephritis. Pharm Res. 2005;22:2016–22.
Khamdang S, Takeda M, Babu E, Noshiro R, Onozato ML, Tojo A, Enomoto A, Huang XL, Narikawa S, Anzai N, Piyachaturawat P, Endou H. Interaction of human and rat organic anion transporter 2 with various cephalosporin antibiotics. Eur J Pharmacol. 2003;465:1–7.
Muck W, Mai I, Fritsche L, Ochmann K, Rohde G, Unger S, Johne A, Bauer S, Budde K, Roots I, Neumayer HH, Kuhlmann J. Increase in cerivastatin systemic exposure after single and multiple dosing in cyclosporine-treated kidney transplant recipients. Clin Pharmacol Ther. 1999;65:251–61.
Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–46.
Tsuruoka S, Ioka T, Wakaumi M, Sakamoto K, Ookami H, Fujimura A. Severe arrhythmia as a result of the interaction of cetirizine and pilsicainide in a patient with renal insufficiency: first case presentation showing competition for excretion via renal multidrug resistance protein 1 and organic cation transporter 2. Clin Pharmacol Ther. 2006;79:389–96.
Jaehde U, Sorgel F, Reiter A, Sigl G, Naber KG, Schunack W. Effect of probenecid on the distribution and elimination of ciprofloxacin in humans. Clin Pharmacol Ther. 1995;58:532–41.
Desrayaud S, Guntz P, Scherrmann JM, Lemaire M. Effect of the P-glycoprotein inhibitor, SDZ PSC 833, on the blood and brain pharmacokinetics of colchicine. Life Sci. 1997;61:153–63.
Speeg KV, Maldonado AL, Liaci J, Muirhead D. Effect of cyclosporine on colchicine secretion by a liver canalicular transporter studied in vivo. Hepatology 1992;15:899–903.
Nooter K, Oostrum R, Deurloo J. Effects of verapamil on the pharmacokinetics of daunomycin in the rat. Cancer Chemother Pharmacol. 1987;20:176–8.
Yesair DW, Schwartzbach E, Shuck D, Denine EP, Asbell MA. Comparative pharmacokinetics of daunomycin and adriamycin in several animal species. Cancer Res. 1972;32:1177–83.
Lam JL, Shugarts SB, Okochi H, Benet LZ. Elucidating the effect of final-day dosing of rifampin in induction studies on hepatic drug disposition and metabolism. J Pharmacol Exp Ther. 2006;319:864–70.
Ding R, Tayrouz Y, Riedel KD, Burhenne J, Weiss J, Mikus G, Haefeli WE. Substantial pharmacokinetic interaction between digoxin and ritonavir in healthy volunteers. Clin Pharmacol Ther. 2004;76:73–84.
Inotsume N, Nishimura M, Nakano M, Fujiyama S, Sato T. The inhibitory effect of probenecid on renal excretion of famotidine in young, healthy volunteers. J Clin Pharmacol. 1990;30:50–6.
Tahara H, Kusuhara H, Chida M, Fuse E, Sugiyama Y. Is the monkey an appropriate animal model to examine drug-drug interactions involving renal clearance? Effect of probenecid on the renal elimination of H2 receptor antagonists. J Pharmacol Exp Ther. 2006;316:1187–94.
Zheng HX, Huang Y, Frassetto LA, Benet LZ. Elucidating rifampin’s inducing and inhibiting effects on glyburide pharmacokinetics and blood glucose in healthy volunteers: unmasking the differential effects of enzyme induction and transporter inhibition for a drug and its primary metabolite. Clin Pharmacol Ther. 2009;85:78–85.
Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–80.
Breedveld P, Zelcer N, Pluim D, Sonmezer O, Tibben MM, Beijnen JH, Schinkel AH, van Tellingen O, Borst P, Schellens JH. Mechanism of the pharmacokinetic interaction between methotrexate and benzimidazoles: potential role for breast cancer resistance protein in clinical drug-drug interactions. Cancer Res. 2004;64:5804–11.
VanWert AL, Bailey RM, Sweet DH. Organic anion transporter 3 (Oat3/Slc22a8) knockout mice exhibit altered clearance and distribution of penicillin G. Am J Physiol Renal Physiol. 2007;293:F1332–F41.
Shiga T, Hashiguchi M, Urae A, Kasanuki H, Rikihisa T. Effect of cimetidine and probenecid on pilsicainide renal clearance in humans. Clin Pharmacol Ther. 2000;67:222–8.
Chung JY, Cho JY, Yu KS, Kim JR, Oh DS, Jung HR, Lim KS, Moon KH, Shin SG, Jang IJ. Effect of OATP1B1 (SLCO1B1) variant alleles on the pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2005;78:342–50.
Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–7.
Bauer LA, Black DJ, Lill JS, Garrison J, Raisys VA, Hooton TM. Levofloxacin and ciprofloxacin decrease procainamide and N-acetylprocainamide renal clearances. Antimicrob Agents Chemother. 2005;49:1649–51.
Somogyi A, McLean A, Heinzow B. Cimetidine-procainamide pharmacokinetic interaction in man: evidence of competition for tubular secretion of basic drugs. Eur J Clin Pharmacol. 1983;25:339–45.
Kajosaari LI, Niemi M, Neuvonen M, Laitila J, Neuvonen PJ, Backman JT. Cyclosporine markedly raises the plasma concentrations of repaglinide. Clin Pharmacol Ther. 2005;78:388–99.
Simonson SG, Raza A, Martin PD, Mitchell PD, Jarcho JA, Brown CD, Windass AS, Schneck DW. Rosuvastatin pharmacokinetics in heart transplant recipients administered an antirejection regimen including cyclosporine. Clin Pharmacol Ther. 2004;76:167–77.
Schneck DW, Birmingham BK, Zalikowski JA, Mitchell PD, Wang Y, Martin PD, Lasseter KC, Brown CD, Windass AS, Raza A. The effect of gemfibrozil on the pharmacokinetics of rosuvastatin. Clin Pharmacol Ther. 2004;75:455–63.
Carr RA, Pasutto FM, Foster RT. Influence of cimetidine coadministration on the pharmacokinetics of sotalol enantiomers in an anaesthetized rat model: evidence supporting active renal excretion of sotalol. Biopharm Drug Dispos. 1996;17:55–69.
Ullrich KJ. Affinity of drugs to the different renal transporters for organic anions and organic cations. In: Amidon GL, Sadee W, editors. Membrane Transporters as Drug Targets. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 159–79.
Yokogawa K, Takahashi M, Tamai I, Konishi H, Nomura M, Moritani S, Miyamoto K, Tsuji A. P-glycoprotein-dependent disposition kinetics of tacrolimus: studies in mdr1a knockout mice. Pharm Res. 1999;16:1213–8.
Bajcetic M, Benndorf RA, Appel D, Schwedhelm E, Schulze F, Riekhof D, Maas R, Boger RH. Pharmacokinetics of oral doses of telmisartan and nisoldipine, given alone and in combination, in patients with essential hypertension. J Clin Pharmacol. 2007;47:295–304.
Oh YH, Han HK. Pharmacokinetic interaction of tetracycline with non-steroidal anti-inflammatory drugs via organic anion transporters in rats. Pharmacol Res. 2006;53:75–9.
Dresser MJ, Leabman MK, Giacomini KM. Transporters involved in the elimination of drugs in the kidney: organic anion transporters and organic cation transporters. J Pharm Sci. 2001;90:397–421.
van Giersbergen PL, Bodin F, Dingemanse J. Cyclosporin increases the exposure to tezosentan, an intravenous dual endothelin receptor antagonist. Eur J Clin Pharmacol. 2002;58:243–5.
Zamboni WC, Houghton PJ, Johnson RK, Hulstein JL, Crom WR, Cheshire PJ, Hanna SK, Richmond LB, Luo X, Stewart CF. Probenecid alters topotecan systemic and renal disposition by inhibiting renal tubular secretion. J Pharmacol Exp Ther. 1998;284:89–94.
Su Y, Hu P, Lee SH, Sinko PJ. Using novobiocin as a specific inhibitor of breast cancer resistant protein to assess the role of transporter in the absorption and disposition of topotecan. J Pharm Pharmaceut Sci. 2007;10:519–36.
Yagi Y, Shibutani S, Hodoshima N, Ishiwata K, Okudaira N, Li Q, Sai Y, Kato Y, Tsuji A. Involvement of multiple transport systems in the disposition of an active metabolite of a prodrug-type new quinolone antibiotic, prulifloxacin. Drug Metab Pharmacokinet. 2003;18:381–9.
Yagi Y, Aoki M, Iguchi M, Shibasaki S, Kurosawa T, Kato Y, Tsuji A. Transporter-mediated hepatic uptake of ulifloxacin, an active metabolite of a prodrug-type new quinolone antibiotic prulifloxacin, in rats. Drug Metab Pharmacokinet. 2007;22:350–7.
Nakashima M, Uematsu T, Kosuge K, Okuyama Y, Morino A, Ozaki M, Takebe Y. Pharmacokinetics and safety of NM441, a new quinolone, in healthy male volunteers. J Clin Pharmacol. 1994;34:930–7.
Yamashiro W, Maeda K, Hirouchi M, Adachi Y, Hu Z, Sugiyama Y. Involvement of transporters in the hepatic uptake and biliary excretion of valsartan, a selective antagonist of the angiotensin II AT1-receptor, in humans. Drug Metab Dispos. 2006;34:1247–54.
Sahin S, Benet LZ. The operational multiple dosing half-life: a key to defining drug accumulation in patients and to designing extended release dosage forms. Pharm Res. 2008;25:2869–77.
Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65.
Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia: Elsevier Saunders; 2006.
Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277:26934–43.
van der Valk P, van Kalken CK, Ketelaars H, Broxterman HJ, Scheffer G, Kuiper CM, Tsuruo T, Lankelma J, Meijer CJ, Pinedo HM. Distribution of multi-drug resistance-associated P-glycoprotein in normal and neoplastic human tissues. Analysis with 3 monoclonal antibodies recognizing different epitopes of the P-glycoprotein molecule. Ann Oncol 1990;1:56–64.
Croop JM, Raymond M, Haber D, Devault A, Arceci RJ, Gros P, Housman DE. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol Cell Biol. 1989;9:1346–50.
Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326:181–7.
Pavlova A, Sakurai H, Leclercq B, Beier DR, Yu AS, Nigam SK. Developmentally regulated expression of organic ion transporters NKT (OAT1), OCT1, NLT (OAT2), and Roct. Am J Physiol Renal Physiol. 2000;278:F635–F43.
Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Eur J Physiol. 2000;440:337–50.
Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275:4507–12.
Kobayashi Y, Ohshiro N, Shibusawa A, Sasaki T, Tokuyama S, Sekine T, Endou H, Yamamoto T. Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in mice. Mol Pharmacol. 2002;62:7–14.
Wang Q, Yang H, Miller DW, Elmquist WF. Effect of the P-glycoprotein inhibitor, cyclosporin A, on the distribution of rhodamine-123 to the brain: an in vivo microdialysis study in freely moving rats. Biochem Biophys Res Commun. 1995;211:719–26.
Kunihara M, Nagai J, Murakami T, Takano M. Renal excretion of rhodamine 123, a P-glycoprotein substrate, in rats with glycerol-induced acute renal failure. J Pharm Pharmacol. 1998;50:1161–5.
Tachibana-Iimori R, Tabara Y, Kusuhara H, Kohara K, Kawamoto R, Nakura J, Tokunaga K, Kondo I, Sugiyama Y, Miki T. Effect of genetic polymorphism of OATP-C (SLCO1B1) on lipid-lowering response to HMG-CoA reductase inhibitors. Drug Metab Pharmacokinet. 2004;19:375–80.
Lin JH, Los LE, Ulm EH, Duggan DE. Kinetic studies on the competition between famotidine and cimetidine in rats. Evidence of multiple renal secretory systems for organic cations. Drug Metab Dispos 1988;16:52–6.
Lau YY. Examining the regulation of hepatic drug disposition and metabolism by organic anion transporting peptide, p-glycoprotein, and multidrug resistance-associated protein 2 [dissertation]. San Francisco: University of California; 2006.
Kugler AR, Olson SC, Smith DE. Tubular transport mechanisms of quinapril and quinaprilat in the isolated perfused rat kidney: effect of organic anions and cations. J Pharmacokinet Biopharm. 1996;24:349–68.
Wu CY. The interactive roles of p-glycoprotein and cytochrome P-450 3A in intestinal and hepatic drug disposition [dissertation]. San Francisco: University of California; 2003.
Booth CL, Brouwer KR, Brouwer KL. Effect of multidrug resistance modulators on the hepatobiliary disposition of doxorubicin in the isolated perfused rat liver. Cancer Res. 1998;58:3641–8.
Acknowledgements
This work was supported in part by NIH grants GM61390 and GM75900, as well as by an unrestricted grant from Amgen, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grover, A., Benet, L.Z. Effects of Drug Transporters on Volume of Distribution. AAPS J 11, 250–261 (2009). https://doi.org/10.1208/s12248-009-9102-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-009-9102-7