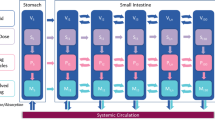
Abstract
Absorption models used in the estimation of pharmacokinetic drug characteristics from plasma concentration data are generally empirical and simple, utilizing no prior information on gastro-intestinal (GI) transit patterns. Our aim was to develop and evaluate an estimation strategy based on a mechanism-based model for drug absorption, which takes into account the tablet movement through the GI transit. This work is an extension of a previous model utilizing tablet movement characteristics derived from magnetic marker monitoring (MMM) and pharmacokinetic data. The new approach, which replaces MMM data with a GI transit model, was evaluated in data sets where MMM data were available (felodipine) or not available (diclofenac). Pharmacokinetic profiles in both datasets were well described by the model according to goodness-of-fit plots. Visual predictive checks showed the model to give superior simulation properties compared with a standard empirical approach (first-order absorption rate + lag-time). This model represents a step towards an integrated mechanism-based NLME model, where the use of physiological knowledge and in vitro–in vivo correlation helps fully characterize PK and generate hypotheses for new formulations or specific populations.







Similar content being viewed by others
References
Huang W, Lee SL, Yu LX. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009;11(2):217–24. doi:10.1208/s12248-009-9098-z.
Hirtz J. The gastrointestinal absorption of drugs in man: a review of current concepts and methods of investigation. Br J Clin Pharmacol. 1985;19 Suppl 2:77S–83S.
Fernandez N, Garcia JJ, Diez MJ, Sahagun AM, Gonzalez A, Diez R, et al. Effects of slowed gastrointestinal motility on levodopa pharmacokinetics. Auton Neurosci. 2010;156(1–2):67–72. doi:10.1016/j.autneu.2010.03.016.
Hardoff R, Sula M, Tamir A, Soil A, Front A, Badarna S, et al. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov Disord. 2001;16(6):1041–7. doi:10.1002/mds.1203.
Yu LX, Amidon GL. A compartmental absorption and transit model for estimating oral drug absorption. Int J Pharm. 1999;186(2):119–25.
Agoram B, Woltosz WS, Bolger MB. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv Drug Deliv Rev. 2001;50 Suppl 1:S41–67.
Jamei M, Marciniak S, Feng K, Barnett A, Tucker G, Rostami-Hodjegan A. The Simcyp((R)) Population-based ADME Simulator. Expert Opin Drug Metab Toxicol. 2009. doi: 10.1517/17425250802691074
Savic RM, Jonker DM, Kerbusch T, Karlsson MO. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn. 2007;34(5):711–26. doi:10.1007/s10928-007-9066-0.
Plusquellec Y, Campistron G, Staveris S, Barre J, Jung L, Tillement JP, et al. A double-peak phenomenon in the pharmacokinetics of veralipride after oral administration: a double-site model for drug absorption. J Pharmacokinet Biopharm. 1987;15(3):225–39.
Plusquellec Y, Efthymiopoulos C, Duthil P, Houin G. A pharmacokinetic model for multiple sites discontinuous gastrointestinal absorption. Med Eng Phys. 1999;21(8):525–32.
Godfrey KR, Arundel PA, Dong Z, Bryant R. Modelling the Double Peak Phenomenon in pharmacokinetics. Comput Methods Programs Biomed. 2010. doi: 10.1016/j.cmpb.2010.03.007
Lindberg-Freijs A, Karlsson MO. Dose dependent absorption and linear disposition of cyclosporin A in rat. Biopharm Drug Dispos. 1994;15(1):75–86.
Weitschies W, Blume H, Monnikes H. Magnetic marker monitoring: high resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur J Pharm Biopharm. 2010;74(1):93–101. doi:10.1016/j.ejpb.2009.07.007.
Bergstrand M, Soderlind E, Weitschies W, Karlsson MO. Mechanistic modeling of a magnetic marker monitoring study linking gastrointestinal tablet transit, in vivo drug release, and pharmacokinetics. Clin Pharmacol Ther. 2009;86(1):77–83. doi:10.1038/clpt.2009.43.
Weitschies W, Kosch O, Monnikes H, Trahms L. Magnetic marker monitoring: an application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms. Adv Drug Deliv Rev. 2005;57(8):1210–22. doi:10.1016/j.addr.2005.01.025.
Weitschies W, Wedemeyer RS, Kosch O, Fach K, Nagel S, Soderlind E, et al. Impact of the intragastric location of extended release tablets on food interactions. J Control Release. 2005;108(2–3):375–85. doi:10.1016/j.jconrel.2005.08.018.
Chan KK, Wnuck K, Bell CL. Microcomputer-based programs for calculating mean and variance residence time by the method of prospective areas. Comput Methods Programs Biomed. 1987;25(1):23–30.
Carlsson KC, Savic RM, Hooker AC, Karlsson MO. Modeling subpopulations with the $MIXTURE subroutine in NONMEM: finding the individual probability of belonging to a subpopulation for the use in model analysis and improved decision making. AAPS J. 2009;11(1):148–54. doi:10.1208/s12248-009-9093-4.
Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33(3):184–213.
Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs. 1980;20(1):24–48.
Standing JF, Howard RF, Johnson A, Savage I, Wong IC. Population pharmacokinetics of oral diclofenac for acute pain in children. Br J Clin Pharmacol. 2008;66(6):846–53. doi:10.1111/j.1365-2125.2008.03289.x.
Korpela R, Olkkola KT. Pharmacokinetics of intravenous diclofenac sodium in children. Eur J Clin Pharmacol. 1990;38(3):293–5.
Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30(5):329–32.
Beal SL, Sheiner LB, Boeckmann A, Bauer RJ. NONMEM User’s Guides (1989–2009). Ellicott City, MD, USA: Icon; 2009.
Lindbom L, Ribbing J, Jonsson EN. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed. 2004;75(2):85–94. doi:10.1016/j.cmpb.2003.11.003.
R DCT. R: A Language and Environment for Statistical Computing. R.2.12.0 ed. Vienna, Austria: R Foundation for Statistical Computing; 2010.
Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58(1):51–64.
Bergstrand M, Karlsson MO. Handling data below the limit of quantification in mixed effect models. AAPS J. 2009;11(2):371–80. doi:10.1208/s12248-009-9112-5.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35(4):401–21. doi:10.1007/s10928-008-9094-4.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504.
Willis JV, Kendall MJ, Flinn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol. 1979;16(6):405–10.
Hénin E, Bergstrand B, Karlsson MO. Meta-analysis of Magnetic Marker Monitoring data to characterize tablet movement through the gastrointestinal tract. PAGE Abstracts of the Annual Meeting of the Population Approach Group in Europe. 2011;PAGE 20 (2011) Abstr 2249 [www.page-meeting.org/?abstract=2249].
Acknowledgments
The authors would like to thank Jeff Rothwell, Rosemont Pharmaceuticals Ltd, UK, and Professor Klaus Olkkola, University of Helsinki, Finland, who provided the diclofenac data. Dr Emilie Henin was supported by a grant to Uppsala University from Novartis, and by the Lavoisier Program from the French Ministry of European Affairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hénin, E., Bergstrand, M., Standing, J.F. et al. A mechanism-Based Approach for Absorption Modeling: The Gastro-Intestinal Transit Time (GITT) Model. AAPS J 14, 155–163 (2012). https://doi.org/10.1208/s12248-012-9324-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-012-9324-y