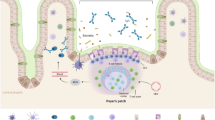
Abstract
Antigens entering the body through the mucosal surface are screened by a highly developed immune system comprised not only of traditional lymphoid cells but also epithelial cells, fibroblasts, and antigen-presenting cells (APCs). For example, in the intestinal tract, gut-associated lymphoid tissue (GALT) is tolerant to the approx 400 separate commensal strains residing mainly in the colon, but also retains the capacity to detect and remove virulent bacteria before they infect systemically. This review summarizes recent work characterizing the molecular mechanisms involved in acute and chronic intestinal inflammation. We will also describe a natural murine pathogen, Citrobacter rodentium, which is being used to explore the host response to enteric pathogens and the resulting immunopathology.
Similar content being viewed by others
References
Mowat AM: Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol 2003;3:331–341.
Sadlack B, Merz H, Schorle H, et al.: Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell 1993;75:253–261.
Kuhn R, Lohler J, Rennick, D, et al.: Interleukin-10 deficient mice develop chronic enterocholitis. Cell 1993;75:263–274.
Mombaerts P, Mizoguchi E, Grusby MJ, et al.: Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 1993;75:274–282.
Fiorucci S, Mencarelli, A, Palazzetti, B, et al.: Importance of innate immunity and collagen binding integrin alphal betal in TNBS-induced colitis. Immunity 2002;17:769–780.
Ogura Y, Bonen DK, Inohara N, et al.: A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 2001;411:603–606.
Strober W, Nakamura K, and Kitani, A: The SAMP1/Yit mouse: another step closer to modeling human inflammatory bowel disease. J Clin Invest 2001;107:667–670.
Taurog JD, Richardson JA, Croft, JT, et al.: The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med 1994;180:2359–2364.
Dianda L, Hanby, AM, Wright, NA, et al.: T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol 1997;150:91–97.
Wei B, Huang T, Dalwadi, H, et al.: Pseudomonas fluorescens encodes the Crohn's disease-associated 12 sequence and T-cell superantigen. Infect Immun 2002;70:6567–6575.
Dalwadi H, Wei, B, Kronenberg, M., et al.: Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun 1999;67:4499–4509.
Glasser AL, Boudeau, J, Barnich, N, et al.: Adherent invasive Escherichia coli strains from patients with Crohn's disease survive and replicate within macrophasges without inducing host cell death. Infect Immun 2001;69:5529–5537.
Kallinowski F, Wassmer, A, Hofmann, MA, et al.: Prevalence of enteropathogenic bacteria in surgically treated chronic inflammatory bowel disease. Hepatogastroenterology 1998;45:1552–1558.
Neut C, Bulois, P, Desreumaux, P, et al.: Changes in the bacterial flora of the neoterminal ileum after ileocolonic resection for Crohn's disease. Am J Gastroenterol 2002;97:939–946.
Neutra MR, Pringault, E and Kraehenbuhl, JP: Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol 1996;14:275–300.
Rescigno M, Urbano, M, Valzasina B, et al.: Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol 2001;2:361–367.
Sabroe I, Parker, LC, Wilson, AG, et al.: Toll-like receptors: their role in allergy and non-allergic inflammatory disease. Clin Exp Allergy 2002;32:984–989.
Abreu MT, Vora, P, Faure, E, et al.: Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proin-flammatory gene expression in response to bacterial lipopolysaccharide. J Immunol 2001;167:1609–1616.
Abreu MT, Arnold, ET, Thomas, LS, et al.: TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem 2002;277:20431–20437.
Gewirtz AT, Navas, TA, Lyons, S, et al.: Cutting edge: bacterial flagellin activates basolaterally expressed tlr5 to induce epithelial proinflammatory gene expression. J Immunol 2001;167:1882–1885.
Ortega-Cava CF, Ishihara, S, Rumi, MA, et al.: Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol 2003;170:3977–3985.
House D, Bishop, A, Parry, C, et al.: Typhoid fever: pathogenesis and disease. Curr Opin Infect Dis 2001;14:573–578.
Wershil BK, Castagliuolo, I and Pothoulakis, C: Direct evidence of mast cell involvement in Clostridium difficile toxin A-induced enteritis in mice. Gastroenterology 1998;114:956–964.
Su B, Ceponis, PJ, Lebel, S, et al.: Helicobacter pylori activates Toll-like receptor 4 expression in gastrointestinal epithelial cells. Infect Immun 2003;71:3496–3502.
Crabtree JE: Role of cytokines in pathogenesis of Helicobacter pylori-induced mucosal damage. Dig Dis Sci 1998;43:46S-55S.
Gionchetti P, Vaira, D, Campieri, M, et al.: Enhanced mucosal interleukin-6 and-8 in Helicobacter pylori-positive dyspeptic patients. Am J Gastroenterol 1994;89:883–887.
Nataro JP and Kaper JB: Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998;11:142–201.
Celli J, Olivier, M, and Finlay, BB: Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J 2001;20:1245–1258.
Stein MA, Mathers, DA, Yan, H, et al.: Enteropathogenic Escherichia coli markedly decreases the resting membrane potential of Caco-2 and HeL a human epithelial cells. Infect Immun 1996;64:4820–4825.
Powrie F, Correa-Oliveira, R, Mauze, S, et al.: Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J Exp Med 1994;179:589–600.
Powrie F, Leach, MW, Mauze, S, et al.: Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol 1993;5:1461–1471.
Pontoux C, Banz, A and Papiernik, M: Natural CD4 CD25(+) regulatory T cells control the burst of super-antigen-induced cytokine production: the role of IL-10. Int Immunol 2002;14:233–239.
Sakaguchi S, Sakaguchi, N, Asano, M, et al.: Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Break-down of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 1995;155:1151–1164.
Wolf M, Schimpl, A and Hunig, T: Control of T cell hyper-activation in IL-2-deficient mice by CD4(+)CD25(-) and CD4(+)CD25(+) T cells: evidence for two distinct regulatory mechanisms. Eur J Immunol 2001;31:1637–1645.
Thornton AM and Shevach EM: CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 1998;188:287–296.
Asano M, Toda M, Sakaguchi N, et al.: Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 1996;184:387–396.
Itoh M, Takahashi T, Sakaguchi N, et al.: Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol 1999;162:5317–5326.
Graca L, Thompson S, Lin, CY, et al.: Both CD4(+) CD25(+) and CD4(+) CD25(−) regulatory cells mediate dominant transplantation tolerance. J Immunol 2002;168: 5558–5565.
Lehmann J, Huehn, J, de la Rosa, M, et al.: Expression of the integrin alpha Ebeta 7 identifies unique subsets of CD25+ as well as CD25− regulatory T cells. Proc Natl Acad Sci USA 2002;99:13031–13036.
Uraushihara K, Kanai, T, Ko, K, et al.: Regulation of murine inflammatory bowel disease by CD25+ and CD25− CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol 2003;171:708–716.
Auernhammer CJ, Bousquet, C and Melmed S: Autoregulation of pituitary corticotroph SOCS-3 expression: characterization of the murine SOCS-3 promoter. Proc Natl Acad Sci USA 1999;96:6964–6969.
Suzuki A, Hanada, T, Mitsuyama, K, et al.: CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J Exp Med 2001;193:471–481.
Fioretino DF, Zlotnik, A, Mosmann, TR, et al.: IL-10 inhibits cytokine production by activated macrophages. J Immunol 1991;147:3815–3822.
D'Andrea A, Aste-Amezaga, M, Valiante, NM, et al.: Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 1993;178:1041–1048.
Gruber MF, Williams, CC and Gerrard, TL: Macrophage-colony-stimulating factor expression by anti-CD45 stimulated human monocytes is transcriptionally up-regulated by IL-1 beta and inhibited by IL-4 and IL-10. J Immunol 1994;152:1354–1361.
Berkman N, John M, Roesems G, et al.: Inhibition of macrophage inflammatory protein-1 alpha expression by IL-10. Differential sensitivities in human blood monocytes and alveolar macrophages. J Immunol 1995;155:4412–4418.
Marfaing-Koka A, Maravic, M, Humbert, M, et al.: Contrasting effects of IL-4, IL-10 and corticosteroids on RANTES production by human monocytes. Int Immunol 1996;8:1587–1594.
Kopydlowski KM, Salkowski, CA, Cody, MJ, et al.: Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol 1999;163:1537–1544.
Muzio M, Bosisio, D, Polentarutti, N, et al.: Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol 2000;164:5998–6004.
Moore KW, de Waal Malefyt, R, Coffman, RL, et al.: Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765.
Lindsay JO, Ciesielski, CJ, Scheinin, T, et al.: Local delivery of adenoviral vectors encoding murine interleukin 10 induces colonic interleukin 10 production and is therapeutic for murine colitis. Gut 2003;52:363–369.
Braat H, Peppelenbosch, MP and Hommes, DW: Interleukin-10-based therapy for inflammatory bowel disease. Expert Opin Biol Ther 2003;3:725–731.
Donnenberg MS: Enteropathogenic Escherichia coli. In Infections of the Gastrointestinal Tract. Blaser M, Smith P, Rardin J, et al. (eds.) Lippincott Williams & Wilkins, Philadelphia, 2002, pp. 595–612.
Mead PS, Slutsker, L, Dietz, V, et al.: Food-related ill-ness and death in the United States. Emerg Infect Dis 1999;5:607–625.
Goffaux F, China, B, Janssen L, et al.: Genotypic characterization of enteropathogenic Escherichia coli (EPEC) isolated in Belgium from dogs and cats. Res Microbiol 2000;151:865–871.
Marches O, Nougayrede, JP, Boullier, S, et al.: Role of tir and intimin in the virulence of rabbit enteropathogenic Escherichia coli serotype O103:H2. Infect Immun 2000;68:2171–2182.
Oswald E, Schmidt, H, Morabito, S, et al.: Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect Immun 2000;68:64–71.
McDaniel TK, Jarvis, KG, Donnenberg, MS, et al.: A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA 1995;92:1664–1668.
McDaniel TK and Kaper JB: A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K-12. Mol Microbiol 1997;23:399–407.
Newman JV, Zabel, BA, Jha, SS, et al.: Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect Immun 1999;67:6019–6025.
Klapproth JM, Donnenberg, MS, Abraham, JM, et al.: Products of enteropathogenic Escherichia coli inhibit lymphocyte activation and lymphokine production. Infect Immun 1995;63:2248–2254.
Klapproth JM, Scaletsky, IC, McNamara, BP, et al.: A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect Immun 2000;68:2148–2155.
Vallance BA, Deng, W, Jacobson, K, et al.: Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect Immun 2003; 71:3443–3453.
Higgins LM, Frankel, G, Connerton, I, et al.: Role of bacterial intimin in colonic hyperplasia and inflammation. Science 1999;285:588–591.
Higgins LM, Frankel, G, Douce, G, et al.: Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun 1999;67: 3031–3039.
Frankel G, Lider, O, Hershkoviz, R, et al.: The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to betal integrins. J. Biol. Chem. 1996;271:20359–20364.
Umar S, Scott, J, Sellin, JH, et al.: Murine colonic mucosa hyperproliferation. I. Elevated CFTR expression and enhanced cAMP-dependent Cl(−) secretion. Am J Physiol Gastrointest Liver Physiol 2000;278: G753-G764.
Salvati VM, Bajaj-Elliott, M, Poulsom, R, et al.: Keratinocyte growth factor and coeliac disease. Gut 2001;49:176–181.
Bajaj-Elliott M, Poulsom, R, Pender, SL, et al.: Interactions between stromal cell—derived keratinocyte growth factor ad epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J Clin Invest 1998;102:1473–1480.
Savkovic SD, Koutsouris, A and Hecht, G: Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monoayers induces transmigration of neutrophils. Infect Immun 1996;64:4480–4487.
Vallance BA, Deng, W, Knodler, LA, et al.: Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect Immun 2002;70:2070–2081.
Simmons CP, Goncalves, NS, Ghaem-Maghami, M, et al.: Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J Immunol 2002;168: 1804–1812.
Vallance BA, Deng, W, De Grado, M, et al.: Modulation of inducible nitric oxide synthase expression by the attaching and effacing bacterial pathogen citrobacter rodentium in infected mice. Infect Immun 2002;70: 6424–6435.
Luperchio SA and Schauer DB: Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect 2001;3:333–340.
Smith JA and Bluestone JA: T cell inactivation and cytokine deviation promoted by anti-CD3 mAbs. Curr Opin Immunol 1997;9:648–654.
Merger M, Viney, JL, Borojevic, R, et al.: Defining the roles of perforin, Fas/FasL, and tumour necrosis factor alpha in T cell induced mucosal damage in the mouse intestine. Gut 2002;51:155–163.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sherman, M.A., Kalman, D. Initiation and resolution of mucosal inflammation. Immunol Res 29, 241–252 (2004). https://doi.org/10.1385/IR:29:1-3:241
Issue Date:
DOI: https://doi.org/10.1385/IR:29:1-3:241