Summary
The absorption of methotrexate following intramuscular injection and oral administration of small doses (>30mg/m2) is rapid and complete, whereas with oral doses in excess of 80mg/m2 absorption is less than complete. Pretreatment with oral neomycin decreases and with kanamycin increases the gastrointestinal absorption of oral methotrexate.
The plasma disposition of methotrexate is multiexponential. Due to differences in sampling schedule and assay methods, widely varying estimates of elimination half-life (tJ/2β of 6 to 69 hours of methotrexate have been reported. The long half-life may either be due to enterohepatic circulation of methotrexate and/or its metabolites or a slow elimination of dihydrofolate reductase (DHFR) bound methotrexate. The plasma clearance of methotrexate following small clinical doses is about 80ml/min, but may become saturated at high doses (20g). During high dose infusions, the peak plasma level is proportional to doses up to 200mg/kg.
Methotrexate is transported across cellular membranes via a carrier-mediated active type process. At high concentrations, when the carrier route is saturated, passive diffusion assumes greater importance.
Methotrexate is not highly bound to plasma proteins (∼50%). However, being highly ionised at physiological pH, the drug does not accumulate in the cerebrospinal fluid to any appreciable extent, necessitating intrathecal administration in the treatment of cerebral and meningeal metastases.
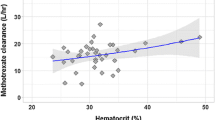
Renal excretion is the major route of elimination for methotrexate (∼80% ); the drug being actively secreted in the renal tubule by the general organic acid transport system. Hence, the renal clearance of methotrexate is decreased by the concomitant administration of organic acids, such as salicylate. The renal clearance of methotrexate is correlated with endogenous creatinine clearance which may provide a guideline to dosage adjustments according to renal function and age. With high dose methotrexate, routine administration of fluid and/or bicarbonate is recommended to prevent intratubular precipitation of the drug.
Biliary excretion of methotrexate constitutes less than 10% of the administered dose. Other extrarenal routes of excretion such as secretion into human breast milk and saliva are negligible.
About a third of an oral dose of methotrexate is metabolised by intestinal bacteria during absorption. The major metabolite is 4-amino-4-deoxy-N10-methylpteroic acid. Small amounts (<11%) of 7 -hydroxymethotrexate have also been found in urine of patients receiving high dose methotrexate therapy. Except for the poly-γ-glutamates, all of the reported metabolites are less effective than methotrexate as an inhibitor of dihydrofolate reductase. As determined by inhibition of DNA synthesis, normal tissues are sensitive to low levels of methotrexate (∼10−8M) Furthermore, toxicity with methotrexate is related to duration of exposure as well as to the dose or plasma concentration.
Impurities, such as methopterin and other byproducts of the synthetic process have been found in commercial parenteral dosage forms of methotrexate. The clinical significance of these impurities requires further study.
For a phase-specific chemotherapeutic agent such as methotrexate, effective plasma levels of the drug should be maintained during the proliferative phase of the tumour cell cycle to achieve a maximum cytotoxic effect. Monitoring the plasma level of methotrexate, particularly during high dose therapy, may provide information regarding impending toxicity and the need for extended citrovorum factor rescue.
Similar content being viewed by others
References
Acute Leukemia Group B: New treatment schedule with improved survival in childhood leukemia. Intermittent parenteral vs daily oral administration of methotrexate for maintenance of induced remission. Journal of American Medical Association 194: 75–81 (1965).
Azarnoff, D.L.; Wan, S.H. and Huffman, D.H.: Pharmacokinetics of methotrexate. Clinical Pharmacology and Therapeutics 16: 884–885 (1974).
Bertino, J.R.; Donohue, D.M.; Simmons, B.; Gabrio, B.W.; Silber, R. and Huennekens, F.M.: The “induction” of dihydrofolic reductase activity in leukocytes and erythrocytes of patients treated with amethopterin. Journal of Clinical Investigation 42: 466–475 (1963).
Bleyer, W.A.; Drake, J.C. and Chabner, B.A.: Neurotoxicity and elevated cerebrospinal fluid methotrexate concentration in meningeal leukemia. New England Journal of Medicine 289: 770–773 (1973).
Baugh, C.M.; Krumdieck, C.L. and Nair, M.G.: Polygammaglutamyl metabolites of methotrexate. Biochemical and Biophysical Research Communications 52: 27–34 (1973).
Bourke, R.S.; Chheda, G.; Brewer, A., Watanabe, O. and Tower, D.B.: Inhibition of renal tubular transport of methotrexate by probenecid. Cancer Research 35: 110–116 (1975).
Capizzi. R.L.; DeConti, R.C.; Marsh, J.C. and Bertino, JR.; Methotrexate therapy of head and neck cancer: improvement in therapeutic index by the use of leucovorin “rescue”. Cancer Research 30: 1782–1788 (1970).
Chabner, B.A. and Young, R.C.: Threshold methotrexate concentration for in vivo inhibition of DNA synthesis in normal and tumorous target tissues. Journal of Clinical Investigation 52: 1804–1811 (1973).
Chabner, B.A. and Slavik, M.: Introduction: Perspectives on high-dose methotrexate (NSC-740) therapy. Cancer Chemotherapy Reports, Part 3, 6: 1–2 (1975).
Cohen, M.H.; Creaven. P.J.; Fossieck, BE.; Johnston, A.V. and Williams, C.L.: Effect of oral prophylactic broad spectrum non-absorbable antibiotics on the gastrointestinal absorption of nutrients and methotrexate in small cell bronchogenic carcinoma patients. Cancer 38: 1556–1559 (1976).
Creaven. P.J.: Hansen. H.H.; Alford. D.A. and Allen. L.M.: Methotrexate in liver and bile after intravenous dosage in man. British Journal of Cancer 28: 589–591 (1973).
Dahl, M.G.; Gregory, M.M. and Scherer, P.J.: Methotrexate hepatotoxicity in psoriasis—comparison of different dose regimens. British Medical Journal 1: 654–656 (1972).
Djerassi, I.; Rominger. C.J.; Kim, J.S.; Turchi, J.; Suvansri, U. and Hughes, D.: Phase I study of high doses of methotrexate with citrovorum factor in patients with lung cancer. Cancer 30: 22–30 (1972).
Djerassi, I.: High-dose methotrexate (NSC-740) and citrovorum factor (NSC-3590) rescue: Background and rationale. Cancer Chemotherapy Reports, Part 3, 6: 3–6 (1975).
Farber. S., Diamond, L.K.; Mercer, R.D.; Sylvester. R.F. and Wolff. J.A.: Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-amino-pteroylglutamic acid (aminopterin). New England Journal of Medicine 238: 787–793 (1948).
Freeman. M.V.: The fluorometric measurement of the absorption, distribution and excretion of single doses of 4-amino-10-methyl-pteroylglutamic acid (amethopterin) in man. Journal of Pharmacology and Experimental Therapeutics 122: 154–162 (1958).
Freeman-Narrod, M.: The pharmacology of methotrexate; in Porter and Wittshaw (Eds) Methotrexate in the Treatment of Cancer, p. 17–21 (Williams and Wilkins, Balitmore 1962).
Freeman-Narrod. M.; Gerstly. B.J.; Engstrom, PF. and Bornstein, R.S.: Comparison of serum concentrations of methotrexate after various routes of administration. Cancer 36: 1619–1624 (1975).
Goldie, JH.; Price, L.A. and Harrap, K.R.: Methotrexate toxicity: Correlation with duration of administration, plasma levels, dose and excretion pattern. European Journal of Cancer 8: 409–414 (1972).
Goldin, A.; Mantel, N.; Greenhouse, S.W.; Venditt, J.M. and Humphrey, SR.: Effect of delayed administration of citrovorum factor on the antileukemic effectiveness of aminopterin in mice. Cancer Research 14: 43–48 (1954).
Goldman, I.D.: The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Annals of the New York Academy of Sciences 186: 400–422 (1971).
Goldman, I.D.: Membrane transport of methotrexate (NSC-740) and other folate compounds: Relevance to rescue protocols. Cancer Chemotherapy Reports, Part 3, 6: 63–72 (1975).
Halprin, K.M.; Fukui, K. and Ohkawara, A.: Blood levels of methotrexate and the treatment of psoriasis. Archives of Dermatology 103: 243–249 (1971).
Henderson, E.S.; Adamson, R.H. and Oliverio, V.T.: The metabolic fate of tritiated methotrexate II. Absorption and excretion in man. Cancer Research 25: 1018–1024 (1965).
Hertz, R.; Lewis, J., Jr. and Lipsett, M.B.: Five year’s experience with the chemotherapy of metastatic choriocarcinoma and related trophoblastic tumor in women. American Journal of Obstetrics and Gynecology 82: 631–640 (1961).
Hignite, C.E.; Shen, D.D. and Azarnoff, D.L.: Separation and identification of impurities in parenteral methotrexate dosage forms. Cancer Treatment Reports. In press (1977).
Hillcoat, B.L.: Swett, V. and Bertino, JR.: Increase of dihydrofolate reductase activity in cultured mammalian cells after exposure to methotrexate. Proceedings of the National Academy of Sciences 58: 1632–1637 (1967).
Ho, D.H.W. and Werkheiser, W.C.: Reappearance of dihydrofolate reductase in rodent tissues following methotrexate administration. Journal of Pharmacokinetics and Biopharmaceutics 3: 265–276 (1975).
Hryniuk, W.M. and Bertino, J.R.: Treatment of leukemia with large doses of methotrexate and folinic acid: clinical-biochemical correlates. Journal of Clinical Investigation 48: 2140–2155 (1969).
Huffman, D.H.; Wan, S.H.; Azarnoff, D.L. and Hoogstraten, B.: Pharmacokinetics of methotrexate. Clinical Pharmacology and Therapeutics 14: 572–579 (1973).
Jacobs, S.A.; Adamson, R.H.; Chabner, B.A.; Derr, C.J. and Johns, D.G.: Stoichiometric inhibition of mammalian dihydrofolate reductase by the γ-glutamyl metabolite of methotrexate, 4-amino-4-deoxy-N10-methyl-pteroylglutamyly-glutamate. Biochemical Biophysical Research Communications 63: 692–698 (1975).
Jacobs, S.A.; Stoller, R.G.; Chabner, B.A. and Johns, D.G.: 7-Hydroxymethotrexate as a urinary metabolite in human subjects and rhesus monkeys receiving high dose methotrexate. Journal of Clinical Investigations 57: 534 (1976).
Jaffe, N.; Frej, E. III; Traggis, D. and Bishop, Y.: Adjuvant methotrexate and citrovourm-factor treatment of osteogenic sarcoma. New England Journal of Medicine 291: 994–997 (1974).
Johns, D.G.; Hollingsworth, J.W.; Cashmore, AR.; Plenderleith, I.H. and Bertino, J.R.: Methotrexate displacement in man. Journal of Clinical Investigation 43: 621–629 (1964).
Johns, D.G. and Loo, T.L.: Metabolite of 4-amino-4-deoxy-N10-methyl pteroylglutamic acid (methotrexate). Journal of Pharmaceutical Sciences 56: 356–359 (1967).
Johns, D.G. and Valerino, D.M.: Metabolism of folate antagonists. Annals of New York Academy of Sciences. 186: 378–386 (1971).
Johns, D.G.; Rutherford. L.D.; Leighton. P.C. and Vogel, C.L.: Secretion of methotrexate into human milk. American Journal of Obstetrics and Gynecology. 112: 978–980 (1972).
Jusko, W.J.: A pharmacodynamic model for cell-cycle-specific chemotherapeutic agents. Journal of Pharmacokinetics and Biopharmaceutics 1: 175–200 (1973).
Kristensen, L.Ø.; Weismann, K. and Hutters, L.: Renal function and the rate of disappearance of methotrexate from serum. European Journal of Clinical Pharmacology. 8: 439–444 (1975).
Leme, P.R.; Creaven, P.J.; Allen, L.M. and Berman, M.: Kinetic model for the disposition and metabolism of moderate and highdose methotrexate (NSC-740) in man. Cancer Chemotherapy Reports, Part 1, 59: 811–817 (1975).
Liegler, D.G.; Henderson, E.S.; Hahn, M.A. and Oliverio, V.T.: The effect of organic acids on renal clearance of methotrexate in man. Clinical Pharmacology and Therapeutics 10: 849–857 (1969).
Liguori, V.R.; Giglio, J.J.; Miller. E. and Sullivan. R.D.: Effects of different dose schedules of amethopterin on serum and tissue concentrations and urinary excretion patterns. Clinical Pharmacology and Therapeutics 3: 34–40 (1962).
Mandel, M.A.: The synergistic effect of salicylates on methotrexate toxicity. Plastic Reconstruction Surgery 57: 733–737 (1976).
Mellett, LB.: Pharmacodynamic and pharmacokinetic measurements of antitumor agents. Clinical Pharmacology and Therapeutics 16: 230–242 (1974).
Naiman, L.J.; Rupprecht, L.M.; Tanyeri, G. and Philippidis, P.: Intrathecal methotrexate. Lancet 1: 571 (1970).
Nirenberg, A.; Masende. C.; Mehta, B.M.; Gisolfi, A.L. and Rosen, G.: High-dose methotrexate with citrovorum factor rescue: predictive value of serum methotrexate concentrations and corrective measures to avert toxicity. Cancer Treatment Reports 61: 779–783 (1977).
Ojima, Y. and Sullivan, R.D.: Pharmacology of methotrexate in the human central nervous system. Surgery, Gynecology and Obstetrics 125: 1035–1040 (1967).
Oliverio, V.T.: Chromatographic separation and purification of folic acid analogs. Analytical Chemistry 33: 263–265 (1961).
Petita, J.S.: Overview of protocols on clinical studies of high-dose methotrexate (NSC-740) with citrovorum factor (NSC-3590) rescue. Cancer Chemotherapy Reports, Part 3, 6: 7–12 (1975).
Pinedo, H.M.; Zaharko. D.S.; Bull, J. and Chabner, B.A.: The relative contribution of drug concentration and duration of exposure to mouse bone marrow toxicity during continuous methotrexate infusion. Cancer Research 37: 445–450 (1977).
Pitman, S.W.; Parker, L.M.; Tattersall, M.H.N.; Jaffe, N. and Frei, E., III: Clinical trial of high-dose methotrexate (NSC-740) with citrovorum factor (NSC-3590)-toxicologic and therapeutic observations. Cancer Chemotherapy Reports, Part 3, 6: 43–49 (1975).
Pratt, C.B.; Roberts, D.; Shanks, E.C. and Warmathy, E.L.: Clinical trials and pharmacokinetics of intermittent highdose methotrexate “leucovorin rescue” for children with malignant tumors. Cancer Research 34: 3326–3331 (1974).
Salassoo, S.; Irving, M.G. and Freedman, A.: Methotrexate megadose followed by folate rescue II. Clearance patterns in patients receiving sequential megadose infusions. Medical Journal of Australia 1: 826–828 (1976).
Shapiro. W.R.; Young, D.F. and Mehta, B.M.: Methotrexate: Distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. New England Journal of Medicine 293: 161–166. (1975).
Skipper, H.E.; Schabel, F.M.; Mellet, L.B.; Montgomery. J.A.; Wilkoff, L.J.; Lloyd, H. and Brockman, R.W.: Implications of biochemical cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Cancer Chemotherapy Reports, Part 1, 54: 431–450 (1970).
Stoller, R.G.; Hard, K.R.; Jacobs, S.A.; Rosenberg, S.A. and Chabner, B.A.: Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. New England Journal of Medicine 297: 630–634 (1977).
Stoller, R.G.; Jacobs, S.A.; Drake, J.C.; Lutz, R.J. and Chabner, B.A.: Pharmacokinetics of high-dose methotrexate (NSC-740). Cancer Chemotherapy Reports, Part 3, 6: 19–24 (1975).
Strum, W.B. and Liem, H.H.: Hepatic uptake intracellular protein binding and biliary excretion of amethopterin. Biochemical Pharmacology 26: 1235–1240 (1977).
Tattersall, M.H.N.; Parker, L.M.; Pitman, S.W. and Frei, E., III.: Clinical Pharmacology of high-dose methotrexate (NSC-740). Cancer Chemotherapy Reports, Part 3, 6: 25–29 (1975).
Tisman, G. and Winsten, W.A.: Methotrexate impurities. Lancet 1: 1178 (1970).
Tong, W.P.; Rosenberg, J. and Ludlum, D.B.: Purity of methotrexate. Lancet 2: 719 (1975).
Valerino, D.M.: Studies of the metabolism of methotrexate II. Isolation and identification of several unconjugated aminop-teridines as metabolites in the rat. Research Communications in Chemical Pathology and Pharmacology 4: 529–542 (1972a).
Valerino, D.M.: Methotrexate impurities. Lancet 2: 1025 (1972b).
Valerino, D.M.; Johns, D.G.; Zaharko. D.S. and Olivero, V.T.: Studies of the metabolism of methotrexate by intestinal flora-I. Identification and study of biological properties of the metabolite 4-amino-4-deoxy-N10-methylpteroic acid. Biochemical Pharmacology 21: 821–831 (1972).
Wan, S.H.; Huffman, D.H.; Azarnoff, D.L.; Stephens, R. and Hoogstraten, B.: Effect of route of administration and effusions on methotrexate pharmacokinetics. Cancer Research 34: 3487–3491 (1974).
Wang, Y.; Kim, P.; van Eys. D.C. and Sutow, W.W.: Study of contaminants and metabolites during therapy with high doses of methotrexate. Clinical Chemistry 22: 1937 (1976).
Weinstein, G.D.: Biochemical and pathophysiological rationale for amethopterin in sporiasis. Annals of the New York Academy of Sciences 186: 452–466 (1971).
Werkheiser, W.C.: Specific binding of 4-amino folic acid analogues by folic acid reductase. Journal of Biological Chemistry 236: 888–893 (1961).
Whitehead, V.M.; Perrault, M.M. and Stelener, S.: Tissue-specific synthesis of methotrexate polyglutamates in the rat. Cancer Research 35: 2985–2990 (1975).
Zaharko, D.S.; Bruckner, H. and Oliverio, V.T.: Antibiotics alter methotrexate metabolism and excretion. Science 166: 887–888 (1969).
Zaharko. D.S.; Dedrick, R.L.: Peale, A.L.; Drake, J.C. and Lutz, R.J.: Relative toxicity of methotrexate in several tissues of mice bearing Lewis Lung carcinoma. Journal of Pharmacology and Experimental Therapeutics 189: 585–592 (1974).
Zurek, W.Z.; Ojima, Y.; Anderson, L.L.; Collins, G.J.; Oberfield, R.A. and Sullivan R.D.: Pharmacologic studies of methotrexate in man. Surgery, Gynecology and Obstetrics 126: 331–338 (1968).
Author information
Authors and Affiliations
Additional information
Supported in part by contract No. 1-CM-12184 and grant GM-15956 from the United States Public Health Service.
Rights and permissions
About this article
Cite this article
Shen, D.D., Azarnoff, D.L. Clinical Pharmacokinetics of Methotrexate. Clin Pharmacokinet 3, 1–13 (1978). https://doi.org/10.2165/00003088-197803010-00001
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-197803010-00001