Abstract
Zafirlukast is a cysteinyl leukotriene type 1 receptor antagonist that causes bronchodilation and has anti-inflammatory properties. Clinical efficacy has been demonstrated when using oral doses of 20 to 40mg twice daily.
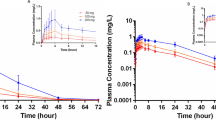
The pharmacokinetics of zafirlukast are best described by a two-compartment model. Maximum plasma concentrations (Cmax) were achieved 3 hours after a single oral dose of 20 or 40mg to healthy volunteers. The absolute bioavailability of zafirlukast is unknown. However, coadministration of zafirlukast with food reduces bioavailability by approximately 40%. The drug binds to plasma proteins (>99%), predominantly to albumin, and has a mean terminal elimination half-life of approximately 10 hours in both healthy volunteers and patients with asthma.
Zafirlukast undergoes extensive hepatic metabolism. Hydroxylation by cytochrome P450 (CYP) 2C9 is the major biotransformation pathway. The metabolites of zafirlukast contribute little to its overall activity. Zafirlukast is mainly eliminated in the faeces, while urinary excretion accounts for <10% of an orally administered dose.
Because of its primarily hepatic metabolism, the clearance of zafirlukast is reduced in patients with hepatic impairment. In patients with stable alcoholic cirrhosis, Cmax and area under the plasma concentration-time curve for zafirlukast were increased by 50 to 60% compared with healthy volunteers. Asymptomatic elevations of serum liver enzymes have been reported with high dosages of zafirlukast (80mg twice daily), returning to normal after cessation of the drug.
Inhibition of the CYP2C9 and CYP3A isoenzymes by zafirlukast has been reported in vitro. Zafirlukast interacts with warfarin and produces a clinically significant increase in the prothrombin time, but it does not alter the pharmacokinetics of terfenadine carboxylate, the active metabolite of terfenadine. Plasma concentrations of zafirlukast decreased when the drug was administered concomitantly with erythromycin, terfenadine or theophylline, and increased when it was coadministered with aspirin (acetylsalicylic acid). Theophylline metabolism is unchanged in most cases by zafirlukast, but there is a report of one patient with increased theophylline plasma concentrations when zafirlukast was coadministered.
Recently, cases of Churg-Strauss syndrome have been described in patients with asthma receiving zafirlukast treatment. This occurrence in patients being withdrawn from corticosteroid therapy while receiving zafirlukast has been attributed to a previously undiagnosed presence of this syndrome in these patients.


Similar content being viewed by others
References
Chanarin N, Johnston SL. Leukotrienes as a target in asthma therapy. Drugs 1994; 47(1): 12–24
Adkins JC, Brogden RN. Zafirlukast: a review of its pharmacology and therapeutic potential in the management of asthma. Drugs 1998; 55(1): 121–44
Busse W. The role and contribution of leukotrienes in asthma. Ann Allergy Asthma Immunol 1998; 81(1): 17–26
Chung KF. Leukotriene receptor antagonists and biosynthesis inhibitors: potential breakthrough in asthma therapy. EurRespir J 1995; 8: 1203–13
Fischer JD, Song MH, Suttle AB, et al. Comparison of zafirlukast (Accolate) absorption after oral and colonic administration in humans. Pharm Res 2000; 17(2): 154–9
Zafirlukast [product monograph]. Lund, Sweden: AstraZeneca Pharmaceuticals, 1999
Savidge RD, Bui KH, Birmingham BK, et al. Metabolism and excretion of zafirlukast in dogs, rats, and mice. Drug Metab Dispos 1998; 26(11): 1069–76
Krell RD, Aharony D, Buckner CK, et al. The preclinical pharmacology of ICI 204,219: a peptide leukotriene antagonist. Am Rev Respir Dis 1990; 141 (4 Pt 1): 978–87
Aharony D. Pharmacology of leukotriene receptor antagonists. Am J Respir Crit Care Med 1998; 157 (6 Pt 2): S214–8
Buckner CK, Fedyna JS, Robertson JL, et al. An examination of the influence of the epithelium on contractile responses to peptidoleukotrienes and blockade by ICI 204,219 in isolated guinea pig trachea and human intralobar airways. J Pharmacol Exp Ther 1990; 252(1): 77–85
Krell RD, Dehaas CJ, Lengel DJ, et al. Preclinical exploration of the potential antiinflammatory properties of the peptide leukotriene antagonist ICI 204,219 (Accolate). Ann NY Acad Sci 1994; 744: 289–98
Spector SL, Smith LJ, Glass M. Effects of 6 weeks of therapy with oral doses of ICI 204,219, a leukotriene D4 receptor antagonist, in subjects with bronchial asthma. ACCOLATE Asthma Trialists Group. Am J Respir Crit Care Med 1994; 150(3): 618–23
Reinus JF, Persky S, Burkiewicz JS, et al. Severe liver injury after treatment with the leukotriene receptor antagonist zafirlukast. Ann Intern Med 2000; 133(12): 964–8
Josefson D. Asthma drug linked with Churg-Strauss syndrome [letter]. BMJ 1997; 315(7104): 330
Churg A, Churg J. Steroids and Churg-Strauss syndrome [letter]. Lancet 1998; 352(9121): 32–3
Wechsler ME, Garpestad E, Flier SR, et al. Pulmonary infiltrates, eosinophilia, and cardiomyopathy following corticosteroid withdrawal in patients with asthma receiving zafirlukast. JAMA 1998; 279(6): 455–7
Holloway J, Ferriss J, Groff J, et al. Churg-Strauss syndrome associated with zafirlukast [published erratum appears in J Am Osteopath Assoc 1998 Dec; 98 (12): 676]. J Am Osteopath Assoc 1998; 98(5): 275–8
Knoell DL, Lucas J, Allen JN. Churg-Strauss syndrome associated with zafirlukast. Chest 1998; 114(1): 332–4
Green RL, Vayonis AG. Churg-Strauss syndrome after zafirlukast in two patients not receiving systemic steroid treatment [letter]. Lancet 1999; 353(9154): 725–6
Wechsler ME, Pauwels R, Drazen JM. Leukotriene modifiers and Churg-Strauss syndrome: adverse effect or response to corticosteroid withdrawal? Drug Saf 1999; 21(4): 241–51
Franco J, Artes MJ. Pulmonary eosinophilia associated with montelukast. Thorax 1999; 54(6): 558–60
Wechsler ME, Finn D, Gunawardena D, et al. Churg-Strauss syndrome in patients receiving montelukast as treatment for asthma. Chest 2000; 117(3): 708–13
Lipworth BJ. Leukotriene-receptor antagonists. Lancet 1999; 353(9146): 57–62
Drazen JM, Israel E, O’Byrne PM. Treatment of asthma with drugs modifying the leukotriene pathway. N Engl J Med 1999; 340(3): 197–206
O’Shaughnessy KM, Taylor IK, O’Connor B, et al. Potent leukotriene D4 receptor antagonist ICI 204,219 given by the inhaled route inhibits the early but not the late phase of allergen-induced bronchoconstriction. Am Rev Respir Dis 1993; 147 (6 Pt 1): 1431–5
Smith LJ, Glass M, Minkwitz MC. Inhibition of leukotriene D4-induced bronchoconstriction in subjects with asthma: a concentration-effect study of ICI 204,219. Clin Pharmacol Ther 1993; 54(4): 430–6
Smith LJ, Hanby LA, Lavins BJ, et al. A single dose of zafirlukast reduces LTD4-induced bronchoconstriction in patients on maintenance inhaled corticosteroid therapy. Ann Allergy Asthma Immunol 1998; 81(1): 43–9
Dessanges JF, Prefaut C, Taytard A, et al. The effect of zafirlukast on repetitive exercise-induced bronchoconstriction: the possible role of leukotrienes in exercise-induced refractoriness. J Allergy Clin Immunol 1999; 104(6): 1155–61
Finnerty JP, Wood BR, Thomson H, et al. Role of leukotrienes in exercise-induced asthma: inhibitory effect of ICI 204219, a potent leukotriene D4 receptor antagonist. Am Rev Respir Dis 1992; 145 (4 Pt 1): 746–9
Coreno A, Skowronski M, Kotaru C, et al. Comparative effects of long-acting beta2-agonists, leukotriene receptor antagonists, and a 5-lipoxygenase inhibitor on exercise-induced asthma. J Allergy Clin Immunol 2000; 106(3): 500–6
Richter K, Jorres RA, Magnussen H. Efficacy and duration of action of the antileukotriene zafirlukast on cold air-induced bronchoconstriction. Eur Respir J 2000; 15(4): 693–9
Taylor IK, O’Shaughnessy KM, Fuller RW, et al. Effect of cysteinyl-leukotriene receptor antagonist ICI 204.219 on allergen-induced bronchoconstriction and airway hyperreactivity in atopic subjects. Lancet 1991; 337(8743): 690–4
Findlay SR, Barden JM, Easley CB, et al. Effect of the oral leukotriene antagonist, ICI 204,219, on antigen-induced bronchoconstriction in subjects with asthma. J Allergy Clin Immunol 1992; 89(5): 1040–5
Dahlen B, Zetterstrom O, Bjorck T, et al. The leukotriene-antagonist ICI-204,219 inhibits the early airway reaction to cumulative bronchial challenge with allergen in atopic asthmatics. Eur Respir J 1994; 7(2): 324–31
Roquet A, Dahlen B, Kumlin M, et al. Combined antagonism of leukotrienes and histamine produces predominant inhibition of allergen-induced early and late phase airway obstruction in asthmatics. Am J Respir Crit Care Med 1997; 155(6): 1856–63
Phipatanakul W, Eggleston PA, Conover-Walker MK, et al. A randomized, double-blind, placebo-controlled trial of the effect of zafirlukast on upper and lower respiratory responses to cat challenge. J Allergy Clin Immunol 2000; 105(4): 704–10
Calhoun WJ, Lavins BJ, Minkwitz MC, et al. Effect of zafirlukast (Accolate) on cellular mediators of inflammation: bronchoalveolar lavage fluid findings after segmental antigen challenge. Am J Respir Crit Care Med 1998; 157 (5 Pt 1): 1381–9
Fish JE, Kemp JP, Lockey RF, et al. Zafirlukast for symptomatic mild-to-moderate asthma: a 13-week multicenter study. The Zafirlukast Trialists Group. Clin Ther 1997; 19(4): 675–90
Suissa S, Dennis R, Ernst P, et al. Effectiveness of the leukotriene receptor antagonist zafirlukast for mild-to-moderate asthma. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1997; 126(3): 177–83
Nathan RA, Bernstein JA, Bielory L, et al. Zafirlukast improves asthma symptoms and quality of life in patients with moderate reversible airflow obstruction. J Allergy Clin Immunol 1998; 102 (6 Pt 1): 935–42
Kemp JP, Minkwitz MC, Bonuccelli CM, et al. Therapeutic effect of zafirlukast as monotherapy in steroid-naive patients with severe persistent asthma. Chest 1999; 115(2): 336–42
Busse W, Nelson H, Wolfe J, et al. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999; 103(6): 1075–80
Nathan RA, Minkwitz MC, Bonuccelli CM. Two first-line therapies in the treatment of mild asthma: use of peak flow variability as a predictor of effectiveness. Ann Allergy Asthma Immunol 1999; 82(5): 497–503
Laitinen LA, Naya I, Binks S. Comparative efficacy of zafirlukast and low dose steroids in asthmatics on prn beta-2-agonists [abstract]. Eur Respir J 1997; 10: S419
Westbroek J, Pasma HR. Effects of 2 weeks of treatment with fluticasone Propionate 100 mcg b.d. by comparison with zafirlukast 20 mg b.d. on bronchial hyper-responsiveness in patients with mild to moderate asthma. Respir Med 2000; 94(2): 112–8
Barnes NC, Miller CJ. Effect of leukotriene receptor antagonisttherapy on the risk of asthma exacerbations in patients with mild to moderate asthma: an integrated analysis of zafirlukast trials. Thorax 2000; 55(6): 478–83
Virchow JC, Prasse A, Naya I, et al. Zafirlukast improves asthma control in patients receiving high-dose inhaled corticosteroids. Am J Respir Crit Care Med 2000; 162 (2 Pt 1): 578–85
Bui KH, Kennedy CM, Azumaya CT, et al. Determination of zafirlukast, a selective leukotriene antagonist, human plasma by normal-phase high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl 1997; 696(1): 131–6
Accolate: zafirlukast [product monograph]. Wilmington (DE): AstraZeneca Pharmaceuticals LP, 2001
Grimm SW, Stams KR, Aaron EJ. Zafirlukast is a substrate for CYP2C9 and an inhibitor of CYP2C9 and CYP3 A in vitro [abstract]. 7th North American International Society for the Study of Xenobiotics Meeting; 1996 Oct 20–25; San Diego, 392
Katial RK, Stelzle RC, Bonner MW, et al. A drug interaction between zafirlukast and theophylline. Arch Intern Med 1998; 158(15): 1713–5
Suttle AB, Vargo DL, Wilkinson LA, et al. Effect of zafirlukast on the pharmacokinetics of R- and S-warfarin in healthy men [abstract]. Clin Pharmacol Ther 1997; 61: 186
Vargo DL, Yeh C, Kane CD, et al. Effect of zafirlukast on prothrombin time and area under the curve of warfarin [abstract]. Allergy 1997; 52: 184
Suttle AB, Birmingham BK, Vargo DL, et al. The pharmacokinetics of zafirlukast and terfenadine after coadministration in healthy men [abstract]. Allergy 1997; 52 Suppl. 37: 184
Vargo DL, Suttle AB, Wilkinson LA, et al. Effect of zafirlukast on QTc and area under the curve of terfenadine in healthy men [abstract]. J Clin Pharmacol 1997; 37: 870
Garey KW, Peloquin CA, Godo PG, et al. Lack of effect of zafirlukast on the pharmacokinetics of azithromycin, clarithromycin, and 14-hydroxyclarithromycin in healthy volunteers. Antimicrob Agents Chemother 1999 May; 43(5): 1152–5
Acknowledgements
The authors did not receive any funding in the preparation of this manuscript. There are no conflicts of interest directly relevant to the contents of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dekhuijzen, P.N.R., Koopmans, P.P. Pharmacokinetic Profile of Zafirlukast. Clin Pharmacokinet 41, 105–114 (2002). https://doi.org/10.2165/00003088-200241020-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200241020-00003