Abstract
Background: The oral hypoglycaemic drug nateglinide is eliminated from the human body via hepatic biotransformation and renal tubular secretion. According to in vitro data, about 70% of nateglinide intrinsic clearance may be mediated by cytochrome P450 (CYP) 2C9 and a smaller fraction by CYP3A4 and CYP2D6. Objective: To assess the impact of CYP2C9 polymorphisms and of the CYP2D6 poor metaboliser genotype on the pharmacokinetics of nateglinide and its effects on insulin, glucose and glucagon in plasma.
Design and participants: A prospective clinical study in 26 healthy volunteers chosen for their CYP2C9 and CYP2D6 genotype was conducted with individuals carrying wild-type genotype as reference group.
Methods: Serial plasma nateglinide, glucose, insulin and glucagon concentrations were measured over 34 hours after a 180mg dose of nateglinide under challenge with 75g of oral glucose at 0, 4 and 8 hours after nateglinide intake. Kinetics were evaluated by nonparametric methods and by population pharmacokinetic-pharmacodynamic modelling.
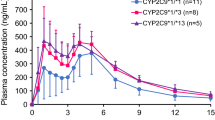
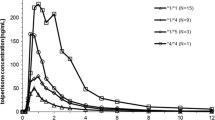
Results: Significantly reduced oral nateglinide clearance was found in carriers of CYP2C9*3 alleles, (p < 0.01), whereas carriers of CYP2C9*2 alleles had kinetic parameters similar to those of carriers of the wild-type allele (p = nonsignificant). Median total clearances were 7.9, 8.4, 6.5, 6.9, 5.8 and 4.1 L/h in carriers of the CYP2C9 genotypes *1/*1, *1/*2, *2/*2, *1/*3, *2/*3 and *3/*3. Median clearance in three carriers of two deficient CYP2D6 alleles was 9.4 L/h. These differences in nateglinide kinetics due to CYP2C9 genotypes did not result in statistically significant differences in plasma glucose, insulin and glucagon. Pharmacokinetic-pharmacodynamic modelling revealed a minor effect of CYP2C9 genotype on insulin and glucose, and extrapolations indicated that carriers of the CYP2C9*3/*3 genotype may be at a slightly higher risk of hypoglycaemia compared with carriers of CYP2C9*1, particularly when taking nateglinide doses above 120mg.
Conclusion: The effect of CYP2C9 polymorphisms on nateglinide kinetics may cause a slightly increased risk for hypoglycaemia, which may become relevant in diabetic patients.









Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Shinkai H, Toi K, Kumashiro I, et al. N-acylphenylalanines and related compounds: a new class of oral hypoglycemic agents. J Med Chem 1988; 31(11): 2092–7
Fujitani S, Yada T. A novel D-phenylalanine-derivative hypoglycemic agent A-4166 increases cytosolic free Ca2+ in rat pancreatic beta-cells by stimulating Ca2+ influx. Endocrinology 1994; 134(3): 1395–400
Akiyoshi M, Kakei M, Nakazaki M, et al. A new hypoglycemic agent, A-4166, inhibits ATP-sensitive potassium channels in rat pancreatic beta-cells. Am J Physiol 1995; 268 (2 Pt 1): E185–93
Marre M, Van Gaal L, Usadel KH, et al. Nateglinide improves glycaemic control when added to metformin monotherapy: results of a randomized trial with type 2 diabetes patients. Diabetes Obes Metab 2002; 4(3): 177–86
Kalbag JB, Walter YH, Nedelman JR, et al. Mealtime glucose regulation with nateglinide in healthy volunteers: comparison with repaglinide and placebo. Diabetes Care 2001; 24(1): 73–7
Kikuchi M. Modulation of insulin secretion in non-insulindependent diabetes mellitus by two novel oral hypoglycaemic agents, NN623 and A4166. Diabet Med 1996; 13 (9 Suppl. 6): S151–5
Hanefeld M, Bouter KP, Dickinson S, et al. Rapid and shortacting mealtime insulin secretion with nateglinide controls both prandial and mean glycemia. Diabetes Care 2000; 23(2): 202–7
Weaver ML, Orwig BA, Rodriguez LC, et al. Pharmacokinetics and metabolism of nateglinide in humans. Drug Metab Dispos 2001; 29(4 Pt 1): 415–21
Takesada H, Matsuda K, Ohtake R, et al. Structure determination of metabolites isolated from urine and bile after administration of AY4166, a novel D-phenylalanine-derivative hypoglycemic agent. Bioorg Med Chem 1996; 4(10): 1771–81
Cao G, Song Y. Pharmacokinetics of enantiomers of a new antidiabetic agent (AY4166) in healthy subjects and its metabolism using isolated rats hepatocytes [abstract]. Clin Pharmacol Ther 2002; 71(2): P100
Hatorp V, Walther KH, Christensen MS, et al. Single-dose pharmacokinetics of repaglinide in subjects with chronic liver disease. J Clin Pharmacol 2000; 40(2): 142–52
Kidd RS, Straughn AB, Meyer MC, et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics 1999; 9(1): 71–80
Kirchheiner J, Bauer S, Meineke l, et al. Impact of CYP2C9 and CYP2C19 polymorphisms on tolbutamide kinetics and on the insulin and glucose response in healthy volunteers. Pharmacogenetics 2002; 12: 101–9
Kirchheiner J, Brockmöller J, Meineke I, et al. Impact of CYP2C9 amino acid polymorphisms on glyburide kinetics and on the insulin and glucose response in healthy volunteers. Clin Pharmacol Ther 2002; 71(4): 286–96
Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics 1996; 6(4): 341–9
Shon JH, Yoon YR, Kim KA, et al. Effects of CYP2C19 and CYP2C9 genetic polymorphisms on the disposition of and blood glucose lowering response to tolbutamide in humans. Pharmacogenetics 2002; 12(2): 111–9
Baldwin SJ, Clarke SE, Chenery RJ. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br J Clin Pharmacol 1999; 48(3): 424–32
Ieiri I, Tainaka H, Morita T, et al. Catalytic activity of three variants (Ile, Leu, and Thr) at amino acid residue 359 in human CYP2C9 gene and simultaneous detection using single-strand conformation polymorphism analysis. Ther Drug Monit 2000; 22(3): 237–44
Stubbins MJ, Harries LW, Smith G, et al. Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics 1996; 6(5): 429–39
Yasar U, Eliasson E, Dahl ML, et al. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun 1999; 254(3): 628–31
Steward DJ, Haining RL, Henne KR, et al. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics 1997; 7(5): 361–7
Furuya H, Fernandez Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics 1995; 5(6): 389–92
Miners JO, Birkett DJ. Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 1998; 45(6): 525–38
Choudhury S, Hirschberg Y, Filipek R, et al. Single-dose pharmacokinetics of nateglinide in subjects with hepatic cirrhosis. J Clin Pharmacol 2000; 40(6): 634–40
Kessler HH, Muhlbauer G, Stelzl E, et al. Fully automated nucleic acid extraction: MagNA Pure LC. Clin Chem 2001; 47(6): 1124–6
Aynacioglu AS, Brockmöller J, Bauer S, et al. Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol 1999; 48(3): 409–15
Sachse C, Brockmöller J, Bauer S, et al. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet 1997; 60(2): 284–95
de Morais SM, Wilkinson GR, Blaisdell J, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem 1994; 269(22): 15419–22
Bauer S, Störmer E, Kirchheiner J, et al. Rapid and simple method for the analysis of nateglinide in human plasma using HPLC analysis with UV detection. J Pharm Biomed Anal 2003; 31(3): 551–5
Kirchheiner J, Meineke I, Müller G, et al. Contributions of CYP2D6, CYP2C9 and CYP2C19 to the biotransformation of E- and Z-doxepin in healthy volunteers. Pharmacogenetics 2002; 12(7): 571–80
Acknowledgements
This study was supported by the German Ministry of Education and Research, BMBF grant no. 01 GG 9845/5 and Grant no. 031U209B. The authors have provided no information on conflicts of interest directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirchheiner, J., Meineke, I., Müller, G. et al. Influence of CYP2C9 and CYP2D6 Polymorphisms on the Pharmacokinetics of Nateglinide in Genotyped Healthy Volunteers. Clin Pharmacokinet 43, 267–278 (2004). https://doi.org/10.2165/00003088-200443040-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200443040-00005