Abstract
Background
Olmesartan medoxomil (CS-866) is a new orally active angiotensin II receptor antagonist that is highly selective for the AT1 receptor subtype.
Objective
To develop a population pharmacokinetic model for olmesartan (RNH-6270), the active metabolite of olmesartan medoxomil, in healthy volunteers and hypertensive patients, and to evaluate effects of covariates on the apparent oral clearance (CL/F), with particular emphasis on the effect of race. Design: Retrospective analysis of data from 12 phase I–III trials in the US, Europe and Japan.
Participants
Eighty-nine healthy volunteers and 383 hypertensive patients.
Methods
Nonlinear mixed-effects modelling was used to evaluate 7911 olmesartan plasma sample concentrations. The covariates included age, bodyweight, sex, race (Westerners [including Caucasians and Hispanics] versus Japanese), patient status (hypertensive patients versus healthy volunteers), serum creatinine level as an index of renal function and serum chemistry data as indices of hepatic function.
Results
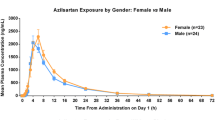
The pharmacokinetic data of olmesartan were well described by a two-compartment linear model with first-order absorption and an absorption lag-time, parameterised in terms of CL/F (6.66 L/h for a typical male Western hypertensive patient), absorption rate constant (1.46h−1), elimination rate constant (0.193h−1), rate constant from the central to peripheral compartment (0.061h−1), rate constant from the peripheral to central compartment (0.079h−1) and absorption lag-time (0.427h). Analysis of covariates showed that age, bodyweight, sex, patient status and renal function were factors influencing the clearance of olmesartan.
Conclusion
The population pharmacokinetic analysis of olmesartan showed that: (i) severe renal impairment (serum creatinine >265 μmol/L [approximately 3 mg/ dL]) could cause a clearance decrease of ≥30%; (ii) older age, lower bodyweight and being female were determinants of lower clearance but their effects on olmesartan clearance were within 20%; (iii) no statistically significant difference in clearance was found between Westerners and Japanese.










Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Mizuno M, Sada T, Ikeda M, et al. Pharmacology of CS-866, a novel nonpeptide angiotensin II receptor antagonist. Eur J Pharmacol 1995; 285(2): 181–8
Koike H, Sada T, Mizuno M. In vitro and in vivo pharmacology of olmesartan medoxomil, an angiotensin II type AT1 receptor antagonist. J Hypertens 2001; 19 Suppl. 1: 3–14
Neutel JM, Elliott WJ, Izzo JL, et al. Antihypertensive efficacy of olmesartan medoxomil, a new angiotensin II receptor antagonist, as assessed by ambulatory blood pressure measurements. J Clin Hypertens 2002; 4(5): 325–31
Neutel JM. Clinical studies of CS-866, the newest angiotensin II receptor antagonist. Am J Cardiol 2001; 87(8A): 37–43
Püchler K, Laeis P, Stumpe KO. Blood pressure response, but not adverse event incidence, correlates with dose of angiotensin II antagonist. J Hypertens Suppl 2001; 19 Suppl. 1: S41–8
Oparil S, Williams D, Chrysant SG, et al. Comparative efficacy of olmesartan, losartan, valsartan and irbesartan in the control of essential hypertension. J Clin Hypertens 2001; 3(5): 283–91
Brunner HR, Klaus O, Stumpe AJ. Antihypertensive efficacy of olmesartan medoxomil and candesartan cilexetil assessed by 24-hour ambulatory blood pressure monitoring in patients with essential hypertension. Clin Drug Invest 2003; 23(7): 419–30
Stumpe KO, Ludwig M. Antihypertensive efficacy of olmesartan compared with other antihypertensive drugs. J Hum Hypertens 2002; 16 Suppl. 2: S24–8
Chrysant SG, Marbury TC, Robinson TD. Antihypertensive efficacy and safety of olmesartan medoxomil compared with amlodipine for mild-to-moderate hypertension. J Hum Hypertens 2003; 17(6): 425–32
Laeis P, Püchler K, Kirch W. The pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interaction. J Hypertens 2001; 19 Suppl. 1: S21–32
Schwocho LR, Masonson HN. Pharmacokinetics of CS-866, a new angiotensin II receptor blocker, in healthy subjects. J Clin Pharmacol 2001; 41(5): 515–27
von Bergmann K, Laeis P, Püchler K, et al. Olmesartan medoxomil: influence of age, renal and hepatic function on the pharmacokinetics of olmesartan medoxomil. J Hypertens 2001; 19 Suppl. 1: S33–40
Csajka C, Buclin T, Fattinger K, et al. Population pharmacokinetic-pharmacodynamic modelling of angiotensin receptor blockade in healthy volunteers. Clin Pharmacokinet 2002; 41(2): 137–52
Guidelines subcommittee. 1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension. Guidelines subcommittee. J Hypertens 1999; 17(2): 151–83
Sone M, Nihei H. Pharmacokinetics of CS-866 (olmesartan medoxomil), an angiotensin II receptor antagonist, in hypertensive patients with renal insufficiency [in Japanese]. Rinshou Iyaku 2004; 20(2): 237–57
Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the Oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60(8): 646–9
Ichikawa S, Takayama Y, Ogihara T. Effects of CS-866 on diurnal blood pressure variation, serum lipid level, hemodynamics, and glucose tolerance in patients with mild-to-moderate essential hypertension [in Japanese]. Rinshou Iyaku 2004; 20(1): 33–60
Tanaka T, Urae R, Hisaoka M, et al. Pharmacokinetics of repeated oral dosages of CS-866 (olmesartan medoxomil), an angiotensin II receptor antagonist, planned to be used clinically [in Japanese]. Rinshou Iyaku 2003; 19(10): 1143–56
Nakashima M, Tanaka T, Osaka A, et al. Investigation of relationship between pharmacokinetics and antihypertensive effect of CS-866 (olmesartan medoxomil) in mild to moderate essential hypertensive patients [in Japanese]. Rinshou iyaku 2003; 19(12): 1397–420
Tanaka T, Urae R, Hisaoka M, et al. Comparison of pharmacokinetics of CS-866 (olmesartan medoxomil), an angiotensin II receptor antagonist, in elderly and non-elderly subjects [in Japanese]. Rinshou Iyaku 2003; 19(11): 1297–306
Kanada S. Pharmacokinetics of 40mg dose of CS-866, an angiotensin II receptor antagonist, in essential hypertensive patients [in Japanese]. Rinshou Iyaku 2003; 19(11): 1271–81
Beal SL, Sheiner LB. NONMEM users’ guides: NONMEM Project Group. San Francisco (CA): University of California at San Francisco, 1998
S-PLUS 6 user’s guide. Seattle (WA): Insightful Corporation, 2001 Jul
Vozeh S, Maitre PO, Stanski DR. Evaluation of population (NONMEM) pharmacokinetic parameter estimates. J Pharmacokinet Biopharm 1990; 18(2): 161–73
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31–41
Johnson JA. Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther 2000; 38(2): 53–60
Mori I, Kazui M, Ikeda T. Binding of 14C-RNH-6270 to serum proteins in vitro. Tokyo: Sankyo Company Ltd, Shinagawa-ku, 1993
Acknowledgements
The authors would like to thank Dr Lewis Sheiner, who sadly passed away in 2004, for his help in reviewing the manuscript.
No sources of funding were used to assist in the preparation of this study. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshihara, K., Gao, Y., Shiga, H. et al. Population Pharmacokinetics of Olmesartan Following Oral Administration of its Prodrug, Olmesartan Medoxomil. Clin Pharmacokinet 44, 1329–1342 (2005). https://doi.org/10.2165/00003088-200544120-00011
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003088-200544120-00011