Summary
Abstract
Exenatide (Byetta™) is a novel, synthetic, incretin mimetic, glucoregulatory peptide approved in the US and Europe for the treatment of patients with type 2 diabetes mellitus who have inadequate glycaemic control despite receiving treatment with maximum tolerated doses of metformin and/or a sulfonylurea. In randomised, controlled, phase III trials and post hoc completer analyses in this patient population, the addition of subcutaneous exenatide twice daily significantly improved glycaemic control and was associated with progressive and significant bodyweight reduction from baseline for up to 2 years. The overall intensity of glycaemic control with exenatide was similar to that achieved with once-daily insulin glargine or twice-daily biphasic insulin aspart. Exenatide was generally well tolerated. Most adverse events were mild to moderate in severity and gastrointestinal in nature. The overall rate of hypoglycaemia was similar to rates observed with placebo (when administered with metformin) and insulin comparators (when administered with metformin and a sulfonylurea). The addition of exenatide to therapy with metformin and a sulfonylurea provided significant improvements in treatment satisfaction and patients’ health-related quality of life (HR-QOL). The drug was also cost effective compared with pioglitazone, glibenclamide (glyburide), insulin glargine (all in combination with metformin and/or a sulfonylurea) and metformin alone. Overall, adjunctive therapy with exenatide is a valuable therapeutic option in patients with type 2 diabetes requiring moderate improvements in glycaemic control despite treatment with metformin and/or a sulfonylurea.
Pharmacological Properties
Exenatide is a synthetic peptide drug with structural and functional similarity to human incretin hormone glucagon-like peptide-1 (GLP-1). Exenatide shares most of the glucoregulatory actions of GLP-1, but unlike GLP-1, is resistant to in vivo proteolytic degradation by dipeptidyl peptidase-IV and has a significantly longer elimination half-life. The insulinotropic activity of exenatide is mediated through stimulation of pancreatic GLP-1 receptors. The glucoregulatory mechanisms of exenatide are (i) acute: enhancement of glucose-dependent insulin secretion, suppression of inappropriately elevated glucagon secretion and slowing of gastric emptying; and (ii) chronic: reduction of food intake and bodyweight and enhanced insulin sensitivity. In patients with type 2 diabetes, twice-daily administration of subcutaneous exenatide causes rapid and significant reduction in fasting and postprandial plasma glucose levels and significant and dose-dependent reduction in glycosylated haemoglobin (HbA1c).
Following subcutaneous administration of exenatide in patients with type 2 diabetes, total drug exposure increases in a dose-proportional manner, whereas increases in peak plasma drug concentrations (Cmax) are less then proportional. Bioavailability of exenatide is similar whether injected into the upper arm, abdomen or thigh. Median Cmax is reached in ≈2 hours. Exenatide has a high apparent volume of distribution. Kidneys are the primary route of elimination and metabolic inactivation of exenatide. Exenatide does not appear to have clinically significant pharmacokinetic interactions with metformin, sulfonylureas or other orally administered drugs commonly used in patients with type 2 diabetes (e.g. paracetamol [acetaminophen], digoxin, lisinopril, lovastatin, warfarin).
Therapeutic Efficacy
In several large, randomised, multicentre, phase III trials in patients with type 2 diabetes who had inadequate glycaemic control despite therapy with metformin and/or a sulfonylurea, the addition of subcutaneous exenatide 5–10μg twice daily resulted in significantly improved glycaemic control and reduced bodyweight.
In three 30-week, triple-blind, phase III trials, the mean change from baseline in HbA1c (primary efficacy endpoint) was significantly better with either exenatide 5μg or 10μg twice daily than with placebo. In each study, HbA1c values in the exenatide treatment arms declined linearly during the first 12 weeks and remained stable thereafter. A statistically significant difference between exenatide and placebo recipients in HbA1c values was seen as early as week 2 or 4 of treatment and was maintained for ≥2 years in the nonblind extension of placebo-controlled, phase III trials. Among patients with baseline HbA1c >7% in each trial, significantly more patients receiving exenatide 5μg or 10μg twice daily than those receiving placebo achieved a target HbA1c of ≤7% at week 30. Likewise, mean changes from baseline in fasting plasma glucose levels at week 30 were significantly better with exenatide 5μg or 10μg twice daily than with placebo in patients also receiving metformin, alone or in combination with a sulfonylurea. In patients receiving concurrent therapy with sulfonylurea alone, a statistically significant improvement in fasting plasma glucose levels was seen only in recipients of exenatide 10μg twice daily. Exenatide also produced a significantly greater reduction from baseline in postprandial plasma glucose levels compared with placebo in subgroups of patients from two trials who underwent a standardised meal tolerance test. The effect was evident as early as week 4 and maintained through week 30 in both trials.
In two nonblind, phase III trials of 26 and 52 weeks’ duration, the efficacy of adjunctive therapy with exenatide 10μg twice daily was similar to that of once-daily insulin glargine or twice-daily biphasic insulin aspart (insulin doses target-titrated) in improving glycaemic control in patients with type 2 diabetes who were inadequately controlled with metformin plus a sulfonylurea. In both trials, exenatide was noninferior to the insulin comparator in achieving the mean reduction from baseline in HbA1c levels (primary efficacy endpoint). Likewise, there were no statistically significant differences between exenatide and either insulin comparator in the proportion of patients achieving a target HbA1c of ≤7% at study endpoints, and between exenatide and biphasic insulin aspart in reducing fasting plasma glucose levels. Insulin glargine was more effective than exenatide in reducing baseline fasting plasma glucose levels at week 26, although both drugs produced clinically relevant effects. As adjunctive therapy in both trials, exenatide provided better postprandial glycaemic control (e.g. lower blood glucose levels after both morning and evening meals) then either insulin glargine or biphasic insulin aspart.
Adjunctive therapy with twice-daily exenatide was associated with significant (compared with placebo) and progressive reduction from baseline in bodyweight that manifested as early as week 2 of treatment, affected the majority of overweight patients in all phase III trials and was sustained for up to 2 years in a nonblind extension of placebo-controlled trials. The weight loss was dose dependent, did not correlate with the occurrence of nausea and appeared directly proportional to patients’ baseline body mass index. By comparison, patients receiving insulin glargine or biphasic insulin aspart progressively gained weight over 26 and 52 weeks in phase III trials.
Exenatide exhibited similar efficacy to insulin glargine in significantly improving the treatment satisfaction and HR-QOL in patients with type 2 diabetes who were receiving metformin plus a sulfonylurea in a 26-week phase III trial. Importantly, exenatide was cost effective compared with pioglitazone, gliben-clamide, insulin glargine (all in combination with metformin and/or a sulfonylurea) and metformin alone, in two pharmacoeconomic analyses from the US and the UK.
Tolerability
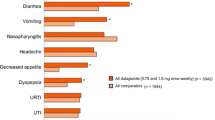
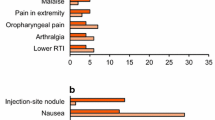
Subcutaneous exenatide 5μg or 10μg twice daily administered in combination with oral metformin and/or sulfonylurea was generally well tolerated in patients with type 2 diabetes in randomised, phase III trials. The majority of treatment-emergent adverse events were mild or moderate in severity, rarely caused treatment discontinuation and, with the exception of hypoglycaemia, were predominantly gastrointestinal in nature. In all trials, nausea was the most common adverse event and occurred more frequently with exenatide (in up to 57% of patients) than with placebo, insulin glargine or biphasic insulin aspart. Hypoglycaemia, which occurred more frequently with exenatide than with placebo only in patients receiving concomitant therapy with a sulfonylurea (with or without metformin), was rarely of clinical significance. The overall rates of hypoglycaemia were low and similar in patients receiving twice-daily exenatide and those receiving once-daily insulin glargine or twice-daily biphasic insulin aspart, all of whom also received metformin plus a sulfonylurea. Exenatide was associated with a significantly lower incidence of nocturnal hypoglycaemia than both insulin comparators, and a significantly higher incidence of daytime hypoglycaemia compared with insulin glargine. The presence of anti-exenatide antibodies (in up to half of all exenatide recipients) had no predictive effect on glycaemic control or adverse events.











Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
UK Prospective Diabetes Study Group. UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 1995 Nov; 44: 1249–58
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998 Sep 12; 352: 837–53
Clemens A, Siegel E, Gallwitz B. Global risk management in type 2 diabetes: blood glucose, blood pressure, and lipids. Update on the background of the current guidelines. Exp Clin Endocrinol Diabetes 2004 Oct; 112 (9): 493–503
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2005 Jan; 28 Suppl. 1: S4–36
American College of Endrocrinology. American College of Endocrinology Consensus Statement on Guidelines for Glycemic Control. Endocrine Practice 2002 Jan–Feb; 8 Suppl. 1: 5–11
Hinnen D, Nielsen LL, Waninger A, et al. Incretin mimetics and DPP-IV inhibitors: new paradigms for the treatment of type 2 diabetes. J Am Board Fam Med 2006 Nov–Dec; 19 (6): 612–20
Keating GM. Exenatide. Drugs 2005; 65 (12): 1681–92; discussion 1693-5
Amylin Pharmaceuticals I. Byetta (exenatide injection): prescribing information [online]. Available from URL: http://www.byetta.com [Accessed 2007 Jan 3]
Copley K, McCowen K, Hiles R, et al. Investigation of exenatide elimination and its in vivo and in vitro degradation. Curr Drug Metab 2006 May; 7 (4): 367–74
Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept 2004; 117: 77–88
Joy SV, Rodgers PT, Scates AC. Incretin mimetics as emerging treatments for type 2 diabetes. Ann Pharmacother 2005 Jan; 39 (1): 110–8
Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006 Nov 11; 368 (9548): 1696–705
Egan JM, Clocquet AR, Elahi D. The insulinotropic effect of acute exendin-4 administered to humans: comparison of nondiabetic state to type 2 diabetes. J Clin Endocrinol Metab 2002 Mar; 87 (3): 1282–90
Degn KB, Brock B, Juhl CB, et al. Effect of intravenous infusion of exenatide (synthetic exendin-4) on glucose-dependent insulin secretion and counterregulation during hypoglycemia. Diabetes 2004 Sep; 53 (9): 2397–403
Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2003 Jul; 88 (7): 3082–9
Fehse F, Trautmann M, Holst JJ, et al. Exenatide augments first and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab 2005 Nov; 90 (11): 5991–7
Blase E, Taylor K, Gao HY, et al. Pharmacokinetics of an oral drug (acetaminophen) administered at various times in relation to subcutaneous injection of exenatide (exendin-4) in healthy subjects. J Clin Pharmacol 2005 May; 45 (5): 570–7
Linnebjerg H, Park S, Kothare P, et al. Exenatide delays gastric emptying and reduces postprandial glucose in type 2 diabetes [abstract no. 0223]. Diabetologia 2006 Sep; 49 Suppl. 1: 140
Kolterman OG, Kim DD, Shen L, et al. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm 2005 Jan 15; 62 (2): 173–81
Rayner CK, Samsom M, Jones KL, et al. Relationship of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001 Feb; 24 (2): 371–81
Bai L, Meredith G, Tuch BE. Glucagon-like peptide-1 enhances production of insulin in insulin-producing cells derived from mouse embryonic stem cells. J Endocrinol 2005 Aug; 186 (2): 343–52
Li L, El-Kholy W, Rhodes CJ, et al. Glucagon-like peptide-1 protects beta cells from cytokine-induced apoptosis and necrosis: role of protein kinase B. Diabetologia 2005 Jul; 48 (7): 1339–49
Gedulin BR, Nikoulina SE, Smith PA, et al. Exenatide (exendin-4) improves insulin sensitivity and ta-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 2005 Apr; 146 (4): 2069–76
Ranta F, Avram D, Berchtold S, et al. Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 2006 May; 55 (5): 1380–90
Chen J, Couto FM, Minn AH, et al. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Commun 2006 Aug 4; 346 (3): 1067–74
Baggio LL, Drucker DJ. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med 2006; 57: 265–81
Gallwitz B. Exenatide in type 2 diabetes: treatment effects in clinical studies and animal study data. Int J Clin Pract 2006 Dec; 60 (12): 1654–61
Alarcon C, Wicksteed B, Rhodes CJ. Exendin 4 controls insulin production in rat islet beta cells predominantly by potentiation of glucose-stimulated proinsulin biosynthesis at the translational level. Diabetologia 2006 Oct 20; 49: 2920–9
Edwards CM, Stanley SA, Davis R, et al. Exendin-4 reduces fasting and postprandial glucose and decreases energy intake in healthy volunteers. Am J Physiol Endocrinol Metab 2001 Jul; 281 (1): E155–61
Linnebjerg H, Kothare PA, Skrivanek Z, et al. Exenatide: effect of injection time on postprandial glucose in patients with Type 2 diabetes. Diabet Med 2006 Mar; 23 (3): 240–5
Poon T, Nelson P, Shen L, et al. Exenatide improves glycemic control and reduces body weight in subjects with type 2 diabetes: a dose-ranging study. Diabetes Technol Ther 2005 Jun; 7 (3): 467–77
Egan JM, Meneilly GS, Elahi D. Effects of 1-mo bolus subcutaneous administration of exendin-4 in type 2 diabetes. Am J Physiol Endocrinol Metab 2003 Jun; 284 (6): E1072–9
Calara F, Taylor K, Han J, et al. A randomized, open-label, crossover study examining the effect of injection site on bioavailability of exenatide (synthetic exendin-4). Clin Ther 2005 Feb; 27 (2): 210–5
Eli Lilly Nederland B.V. Byetta (exenatide) 5 microgram solution for injection, prefilled pen: summary of product characteristics [online]. Available from URL: http://www.elililly.com [Accessed 2007 Jan 3]
Hiles RA, Bawdon RE, Petrella EM. Ex vivo human placental transfer of the peptides pramlintide and exenatide (synthetic exendin-4). Hum Exp Toxicol 2003 Dec; 22 (12): 623–8
Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia 2006 Apr; 49 (4): 706–12
Linnebjerg H, Kothare P, Park S, et al. Exenatide pharmacokinetics in patients with mild to moderate renal dysfunction and end stage renal disease [abstract no. 469-P]. Diabetes 2005; 54 Suppl. 1: A116
Kothare PA, Soon DKW, Linnebjerg H, et al. Effect of exenatide on the steady-state pharmacokinetics of digoxin. J Clin Pharmacol 2005 Sep; 45 (9): 1032–7
Linnebjerg H, Kothare P, Skrivanek Z, et al. Effects of exenatide on statin single-dose pharmacokinetics and 30-week lipid response. Diabet Med 2006 Mar 1; 23 Suppl. 2: 43–4
Kothare P, Linnebjerg H, Shrivanek Z, et al. Effects of exenatide on statin single-dose pharmacokinetics and 30-week lipid response [abstract no. PII-39]. Clin Pharmacol Ther 2006 Feb 1; 79 (2): 46
Soon D, Kothare PA, Linnebjerg H, et al. Effect of exenatide on the pharmacokinetics and pharmacodynamics of warfarin in healthy Asian men. J Clin Pharmacol 2006 Oct; 46 (10): 1179–87
Kothare P, Linnebjerg H, Atkins M, et al. Effect of exenatide on lisinopril pharmacodynamcis in patients treated for hypertension [abstract no. PI-24]. Clin Pharmacol Ther 2005 Feb; 77 (2): P14
Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004 Nov; 27 (11): 2628–35
DeFronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005 May; 28 (5): 1092–100
Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005 May; 28 (5): 1083–91
Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005 Oct 18; 143 (8): 559–69
Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007 Feb; 50 (2): 259–67
Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 2006 Jul 1; 8 (4): 436–47
Buse JB, Klonoff DC, Nielsen LL, et al. Metabolic effects of two years of exenatide treatment on diabetes, obesity, and hepatic biomarkers in patients with type 2 diabetes: An interim analysis of data from the open-label, uncontrolled extension of three double-blind, placebo-controlled trials. Clin Ther 2007 Jan; 29 (1): 139–53
Boye KS, Matza LS, Oglesby A, et al. Patient-reported outcomes in a trial of exenatide and insulin glargine for the treatment of type 2 diabetes. Health Qual Life Outcomes 2006 Oct 11; 4 (80): 1–8
Watkins JB, Minshall ME, Sullivan SD. Application of economic analyses in U.S. imanaged care formulary decisions: a private payer’s experience. J Manag Care Pharm 2006 Nov/Dec; 12 (9): 726–35
Ray JA, Boye KS, Yurgin N, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin 2007 Mar; 23 (3): 609–22
Exenatide scientific slides [compact disk]. Amylin Pharmaceuticals, Inc. and Eli Lilly and Company 2006
National Institute for Clinical Excellence. Management of type 2 diabetes: management of blood glucose [online]. Available from URL: http://www.nice.org.uk [Accessed 2007 Jan 12]
Gallwitz B. Glucagon-like peptide-1-based therapies for the treatment of type 2 diabetes mellitus. Treat Endocrinol 2005; 4 (6): 361–70
Triplitt C, Wright A, Chiquette E. Incretin mimetics and dipeptidyl peptidase-IV inhibitors: potential new therapies for type 2 diabetes mellitus. Pharmacotherapy 2006 Mar; 26 (3): 360–74
Yoo BK, Triller DM, Yoo DJ. Exenatide: a new option for the treatment of type 2 diabetes. Ann Pharmacother 2006 Oct; 40 (10): 1777–84
Taylor JR, Campbell KM. Diabetes drug update: 4 new options and how they stack up. J Fam Pract 2007 Mar; 56 (3): 207–15
Fineman MS, Shen LZ, Taylor K, et al. Effectiveness of progressive dose-escalation of exenatide (exendin-4) in reducing dose-limiting side effects in subjects with type 2 diabetes. Diabetes Metab Res Rev 2004 Sep; 20 (5): 411–7
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: S. Bloom, Department of Metabolic Medicine, Division of Investigative Science, Imperial College London, Hammersmith Hospital, London, UK; R.K. Campbell, Washington State University, Pullman, Washington, USA; D. Elahi, Department of Surgery, Surgical Endocrinology & Metabolism Laboratory, The John Hopkins University, John Hopkins Bayview Medical Center, Baltimore, Maryland, USA; B. Gallwitz, Department of Medicine IV, Eberhard-Karls-University, Tübingen, Germany; D. Hinnen, Mid America Diabetes Associates, Wichita, Kansas, USA; U. Kabadi, Department of Internal Medicine, Division of Endocrinology, University of Iowa College of Medicine, Iowa City, Iowa, USA; B. Tuch, Diabetes Transplant Unit, University of New South Wales and Prince of Wales Hospital, Sydney, New South Wales, Australia.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘exenatide’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘exenatide’. Searches were last updated on 19 March 2007.
Selection: Studies in patients with type 2 diabetes mellitus who received exenatide in addition to metformin and/or a sulfonylurea. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Exenatide, incretin mimetic, type 2 diabetes mellitus, pharmacoeconomics, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability, treatment satisfaction, health-related quality of life.
Rights and permissions
About this article
Cite this article
Cvetković, R.S., Plosker, G.L. Exenatide. Drugs 67, 935–954 (2007). https://doi.org/10.2165/00003495-200767060-00008
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200767060-00008