Abstract
Background and objective: Midostaurin, a novel potent inhibitor of protein kinase C enzyme and class III receptor tyrosine kinases, including Fms-like tyrosine kinase-3 (FLT3) and c-KIT, shows time-dependent pharmacokinetics in human subjects, presumably due to enzyme auto-induction. The purpose of this study was to develop a mechanism-based population pharmacokinetic model to describe the plasma concentration profiles of midostaurin and its metabolites and to characterize the time course of auto-induction.
Subjects and methods: Data from 37 diabetic patients who received oral doses of midostaurin (25 mg twice daily, 50 mg twice daily or 75 mg twice daily) for 28 days were analysed using nonlinear mixed-effects modelling. The structural model included a gut compartment for drug input and central and peripheral compartments for midostaurin, with drug output from the central compartment to either of two compartments for the midostaurin metabolites CGP62221 and CGP52421. Different enzyme induction sub-models were evaluated to account for the observed time-dependent decrease in midostaurin concentrations.
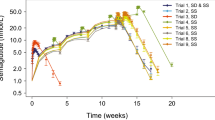
Results: An enzyme turnover model, with CGP62221 formation (CL1) being a linear process but CGP52421 formation (CL2) being inducible, was found to be most appropriate. In the pre-induced state, CL1 and CL2 of midostaurin were determined to be 1.47 L/h and 0.501 L/h, respectively. At the end of 28 days of dosing, CL2 was increased by 5.2-, 6.6- and 6.9-fold in the 25 mg, 50 mg and 75 mg groups, respectively, resulting in a 2.1- to 2.5-fold increase in total clearance of midostaurin. The final model estimated a mean maximum fold of induction (Emax) of 8.61 and a concentration producing 50% of the Emax (EC50) of 1700 ng/mL (∼2.9 μmol/L) for CGP52421-mediated enzyme induction.
Conclusions: The population pharmacokinetic model that was developed was able to describe the time-dependent pharmacokinetic profiles of midostaurin and its auto-induction mechanism. Thus it may be useful for designing an appropriate dosage regimen for midostaurin. The unique feature of this model included a precursor compartment that was able to capture the time delays of auto-induction. The use of such precursor extension in the model may be applicable to other drugs showing long time delays in enzyme auto-induction.








Similar content being viewed by others
References:
Meggio F, Donella Deana A, Ruzzene M, et al. Different susceptibility of protein kinases to staurosporine inhibition: kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem 1995; 234: 317–22
Meyer T, Regenass U, Fabbro D, et al. A derivative of staurosporine (CGP41251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer 1989; 43: 851–6
Andrejauskas-Buchdunger E, Regenass U. Differential inhibition of the epidermal growth factor-, platelet-derived growth factor-, and protein kinase C-mediated signal transduction pathways by the staurosporine derivative CGP 41251. Cancer Res 1992; 52: 5353–8
Fabbro D, Ruetz S, Bodis S, et al. PKC412: a protein kinase inhibitor with a broad therapeutic potential. Anticancer Drug Des 2000; 15: 17–28
Stone RM, DeAngelo DJ, Klimek V, et al. Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 2005; 105: 54–60
Hammes HP, Lin J, Bretzel RG, et al. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes 1998; 47: 401–6
Caldwell RB, Bartoli M, Behzadian MA, et al. Vascular endothelial growth factor and diabetic retinopathy: pathophysiological mechanisms and treatment perspectives. Diabetes Metab Res Rev 2003; 19: 442–55
Seo MS, Kwak N, Ozaki H, et al. Dramatic inhibition of retinal and choroidal neovascularization by oral administration of a kinase inhibitor. Am J Pathol 1999; 154: 1743–53
Saishin Y, Saishin Y, Takahashi K, et al. The kinase inhibitor PKC412 suppresses epiretinal membrane formation and retinal detachment in mice with proliferative retinopathies. Invest Ophthalmol Vis Sci 2003; 44: 3656–62
Campochiaro PA. C99-PKC412-003 Study Group. Reduction of diabetic macular edema by oral administration of the kinase inhibitor PKC412. Invest Ophthalmol Vis Sci 2004; 45: 922–31
Propper DJ, McDonald AC, Man A, et al. Phase I and pharmacokinetic study of PKC412, an inhibitor of protein kinase C. J Clin Oncol 2001; 19: 1485–92
He H, Tran P, Balez S, et al. An open-label single center study to assess PKC412 absorption, distribution, metabolism, and excretion (ADME) after a single oral administration of 50 mg [14C] PKC412 in healthy subjects. East Hanover (NJ): Novartis Pharmaceuticals Corporation, 2005. (Data on file)
Einolf HJ, Bedi Singh S. Evaluation of PKC412 and its metabolites, CGP52421 and CGP62221, to activate the human PXR in a CYP3A4 reporter gene assay. East Hanover (NJ): Novartis Pharmaceuticals Corporation, 2004. (Data on file)
Beal SL, Sheiner LB. NONMEM Project Group. NONMEM® users’ guide. San Francisco (CA): University of California at San Francisco, 1998
Asimus S, Gordi T. Retrospective analysis of artemisinin pharmacokinetics: application of a semiphysiological autoinduction model. Br J Clin Pharmacol 2006; 63: 758–62
Mager DE, Jusko WJ. Pharmacodynamic modeling of time-dependent transduction system. Clin Pharmacol Ther 2001; 70: 210–6
Gabrielsson J, Weiner D. PK/PD data analysis: concepts and applications. 2nd ed. Stockholm: Swedish Pharmaceutical Press; 1998: 450–67
Meibohm B, Derendorf H, Mollmann HW, et al. Mechanism-based PK/PD model for the lymphocytopenia induced by endogenous and exogenous corticosteroids. Int J Clin Pharmacol Ther 1999; 37: 367–76
Yano Y, Beal SL, Sheiner LB. Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 2001; 28: 171–92
Lehmann JM, Mckee DD, Watson MA, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 1998; 102: 1016–23
Bertilsson G, Heidrich J, Svensson K, et al. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A 1998; 95: 12208–13
Levy RH. Time-dependent pharmacokinetics. In: Rowland M, Tucker GT. Pharmacokinetics: theory and methodology. Oxford: Pergamon Press, 1986: 115–30
Warren Jr JW, Benmaman JB, Wannamaker BB, et al. Kinetics of a carbamazepine-ethosuximide interaction. Clin Pharmacol Ther 1980; 28: 646–51
Rostami-Hodjegan A, Wolff K, Hay AWM, et al. Population pharmacokinetics of methadone in opiate users: characterization of time-dependent changes. Br J Clin Pharmacol 1999; 48: 43–52
Von Bahr C, Steiner E, Koike Y, et al. Time course of enzyme induction in humans: effect of pentobarbital on nortriptyline metabolism. Clin Pharmacol Ther 1998; 64: 18–26
Boddy AV, Cole M, Pearson ADJ, et al. The kinetics of the auto-induction of ifosfamide metabolism during continuous infusion. Cancer Chemother Pharmacol 1995; 36: 53–60
Hussein Z, Posner J. Population pharmacokinetics of lamotrigine monotherapy in patients with epilepsy: retrospective analysis of routine monitoring data. Br J Clin Pharmacol 1997; 43: 457–65
Russo H, Dubboin MP, Bressolle F, et al. Time-dependent pharmacokinetics of high dose thiopental infusion in intensive care patients. Pharm Res 1997; 14: 1583–8
Hassan M, Svensson USH, Ljungman P, et al. A mechanism-based pharmacokinetic-enzyme model for cyclophosphamide autoinduction in breast cancer patients. Br J Clin Pharmacol 1999; 48: 669–77
de Jonge ME, Huitema AD, Rodenhuis S, et al. Integrated population pharmacokinetic model of both cyclophosphamide and thiotepa suggesting a mutual drug-drug interaction. J Pharmacokinet Pharmacodyn 2004; 31: 135–56
Acknowledgements
The authors thank members of the nursing and research staff who participated in the clinical study, especially Drs Ken Green, Frances Kane and Peter Graf at CIBA Vision Corporation and Drs Irving E. Weston and James C. Kisicki at MDS Harris. The authors also thank all members of the midostaurin international project team for reviewing the manuscript.
The study was supported by Novartis Pharmaceuticals Corporation. All authors are employees of Novartis Pharmaceuticals Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, O.Q.P., Wang, Y. & Schran, H. A Mechanism-Based Population Pharmacokinetic Model for Characterizing Time-Dependent Pharmacokinetics of Midostaurin and its Metabolites in Human Subjects. Clin Pharmacokinet 47, 807–816 (2008). https://doi.org/10.2165/0003088-200847120-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/0003088-200847120-00005