Summary
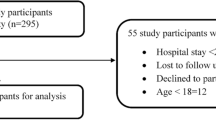
The objective of the present study was to determine the frequency of adverse drug reactions (ADRs) in intensive care units (ICUs) and to evaluate their effect on the length of stay. We performed a prospective study to detect ADRs in 420 patients hospitalised in 10 predetermined beds in the ICU of our hospital between the months of March and December 1996. While the patients were staying in the ICU, data was gathered regarding suspected ADRs and on different variables related to the length of stay. 96 different ADRs were detected in 85 of the 420 patients seen [20.2%, 95% confidence intervals (95% CI) 16.5 to 24.4]. The ADRs were most frequently caused by the following drugs: nitrates (n = 25), opiates (n = 21) and ultrashort-acting benzodiazepines (n = 10). Eight ADRs were severe, the suspected medication had to be discontinued in 51 cases and new drugs were necessary to manage the ADRs in 73 cases. The crude estimation of the effect of the number of ADRs performed with a bivariant regression model indicated that each ADR was related to a 2.38-day increase (95% CI 1.31 to 3.45) in the length of stay. Although this estimation was reduced to 1.76 days (95% CI 0.72 to 2.79), when other confounding variables associated with the length of stay were considered, it was still important.
In conclusion, the ADRs were a significant clinical problem in the ICUs and were responsible for a significant increase in the length of stay.
Similar content being viewed by others
References
Mulroy R. Iatrogenic disease in general practice: its incidence and effects. BMJ 1973; 2: 407–10
Hutchinson TA, Flegel KM, Kramer MS, et al. Frequency, severity and risk factors for adverse drug reactions in adult outpatients: a prospective study. J Chron Dis 1986; 39: 533–42
Spriet A, Spriet C, Larousse C, et al. Methodology and results of a survey of adverse reactions to a drug in private practice. Eur J Clin Pharmacol 1977; 11: 181–92
Martys CR. Adverse reactions to drugs in general practice. BMJ 1979; 2: 1194–7
Abajo FJ, Frías J, Lopo CR, et al. Las reacciones adversas a medicamentos como motivo de consulta al servicio de urgencias de un hospital general [in Spanish]. Med Clin (Barc) 1989; 92: 530–5
Hallas J, Gram L, Grodum E, et al. Drug related admissions to medical wards: a population based survey. Br J Clin Pharmacol 1992; 33: 61–8
Cooke DI, Van der Merwe W, Pudifin DJ. Hospital admissions for adverse reactions to drugs and deliberate self-poisoning. S Afr Med J 1985; 67: 770–2
Caranasos GJ, Stewart RB, Cluff LE. Drug-induced illness leading to hospitalization. JAMA 1974; 228: 713–7
Rostin M, Pascaud A, Lauque D, et al. An intensive survey of pharmacovigilance in a medical admission department. Rev Med Interne 1987; 8: 173–9
Hallas J, Davidsen F, Grodum E, et al. Drug-related illness as a cause of admission to a department of respiratory medicine. Respiration 1992; 59: 30–4
Hallas J, Haghfelt T, Gram LF, et al. Drug related admissions to a cardiology department; frequency and availability. J Intern Med 1990; 228: 379–84
Lakshmanan MG, Hershey CO, Breslau D. Hospital admissions caused by iatrogenic disease. Arch Intern Med 1986; 146: 1931–4
Einarson T. Drug-related hospital admissions. Ann Pharmacother 1993; 27: 832–40
Tiberio G, Hueto J, Moreno M, et al. Reacciones adversas medicamentosas: algoritmos de Naranjo y Venulet [in Spanish]. Rev Clin Esp 1992; 191: 270–3
Diez JL, Muñoz JL, Castro S. Patología iatrogénica en un servicio de medicina interna. IL Reacciones adversas a medicamentos [in Spanish]. Med Clin (Barc) 1986; 87: 131–4
Arnau JM, Camps A, Curull V, et al. Programa de detecciön de reacciones adversas a medicamentos en pacientes hospitalizados. Métodos y resultados de la fase piloto [in Spanish]. Med Clin (Barc) 1984; 82: 433–7
Asscher AW, Parr GD, Whitmarsh VB. Towards the safer use of medicines. BMJ 1995; 311: 1003–5
Davies DM. Adverse drug reactions. J Prac Pharm 1987; 5: 127–32
Danielson DA, Porter JB, Dinan BJ, et al. Drug monitoring of surgical patients. JAMA 1982; 248: 1482–5
Böttiger LE, Furhoff AK, Holmberg L. Fatal reactions to drugs. Acta Med Scand 1979; 205: 451–6
Caranasos GJ, May FE, Stewart RB, et al. Drug-associated deaths of medical inpatients. Arch Intern Med 1976; 136: 872–5
Irey NS. Adverse drug reactions and death. JAMA 1976; 236: 575–8
Girdwood RH. Death after taking medicaments. BMJ 1974; 1: 501–4
Shapiro S, Slone D, Lewis GP, et al. Fatal drug reactions among medical inpatients. JAMA 1971; 216: 467–72
Armstrong B, Dinan B, Jick H. Fatal drug reactions in patients admitted to surgical services. Am J Surg 1976; 132: 643–5
Porter J, Jick H. Drug-related deaths among medical inpatients. JAMA 1977; 237: 879–81
Schneider PJ, Gift MG, Lee Y, et al. Cost of medication-related problems at a university hospital. Am J Health-Sys Pharm 1995; 52: 2415–8
Moore ND, Lecointre D, Noblet C, et al. Serious adverse drug reactions in a Department of Internal Medicine: incidence and cost analysis. Pharmacoepidemiol Drug Saf 1995; 5Suppl. 1: 74
Johnson JA, Bootman JL. Drug-related morbidity and mortality. Acost-of-illness model. Arch Intern Med 1995; 155: 1949–56
Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients. JAMA 1997; 277: 307–11
Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277: 301–6
Knaus WA, Wagner DP, Draper EA, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–29
Karch FE, Lasagna L. Adverse drug reactions. A critical review. JAMA 1975; 234: 1236–41
Venulet J. Methods of monitoring adverse reactions to drugs. In: E. Juscken, editor. Progress in drug research. Basilea: Berghausen Verlag, 1977: 277–84
Naranjo CA, Busto U, Sellers EM, et al. A method for estimating probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–45
International Classification of Diseases, 9th rev. Clinical Modification (ICD-9-CM). Commission on Professional and Hospital Activities, Ann Arbor, Michigan, 1990
Falk RH. Adverse reactions to medication on a coronary care unit. Postgrad Med 1979; 55: 870–3
Italian Group on Intensive Care Evaluation. Epidemiology of adverse drug reactions in intensive care units. A multicentre prospective survey. Eur J Clin Pharmacol 1987; 31: 507–12
Knaus WA, Wagner DP, Zimmerman JE, et al. Variations in mortality and length of stay in intensive care units. Ann Intern Med 1993; 118: 753–61
Rué M, Roqué M, Mestre J, et al. Mortalidad y estancia hospitalaria ajustada por gravedad como indicadores de efectividad y eficacia de la atención a pacientes en estado crítico [in Spanish]. Med Clin (Barc) 1997; 108: 647–51
Llodra-Calvo JC; Vázquez-Mata G, Bueno-Cavanillas A, et al. Valoración del coste de una unidad de medicina intensiva. Relación del coste con la gravedad del enfermo [in Spanish]. Med Clin (Barc) 1994; 103: 49–53
Hurwitz N. Predisposing factors in adverse reactions to drugs. BMJ 1969; 1: 536–9
Klein U, Klein M, Sturm H, et al. The frequency of adverse drug reactions as dependent upon age, sex and duration of hospitalization. Int J Clin Pharmacol 1976; 13: 187–95
Spino M, Sellers EM, Kaplan HL. Effect of adverse drug reactions on the length of hospitalization. Am J Hosp Pharm 1978; 35: 1060–4
Evans RS, Classen DC, Stevens LE, et al. Using a hospital information system to assess the effects of adverse drug events. Pro Annu Comput Appl Med Care 1993; 17: 161–5
Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA 1995; 24: 29–34
Leape LL, Lawthers AG, Brennan TA, et al. Preventing medical injury. Qual Rev Bull 1993; 19: 144–9
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vargas, E., Simón, J., Martin, JC. et al. Effect of Adverse Drug Reactions on Length of Stay in Intensive Care Units. Clin. Drug Investig. 15, 353–360 (1998). https://doi.org/10.2165/00044011-199815040-00011
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199815040-00011