Abstract
Background and objective: Most proton pump inhibitors are extensively metabolized by cytochrome P450 (CYP) isoenzymes, as are many other drugs, giving rise to potential drug-drug interactions. Dexlansoprazole modified release (MR) [TAK-390MR] is a modified-release formulation of dexlansoprazole (TAK-390), an enantiomer of lansoprazole, which employs an innovative Dual Delayed Release™ technology designed to prolong the plasma dexlansoprazole concentration-time profile following once-daily oral administration. As with lansoprazole, dexlansoprazole is metabolized mainly by CYP3A and CYP2C19. Based on in vitro studies, dexlansoprazole has the potential to inhibit activity of these isoenzymes and also may induce human hepatic CYP1A and CYP2C9 activity. To determine whether dexlansoprazole has an effect on these isoenzymes in vivo, drug interaction studies with dexlansoprazole MR were conducted.
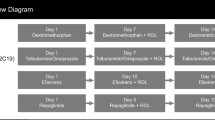
Methods: Four separate randomized, double-blind, two-way crossover, placebo-controlled, single-centre studies were conducted in healthy volunteers to evaluate the effect of dexlansoprazole on the pharmacokinetics of four test substrates (diazepam, phenytoin, theophylline [administered as intravenous aminophylline] and warfarin), which were selected based on in vitro and/or in vivo data that suggest a potential drug interaction with CYP isoenzymes or potentially coadministered narrow therapeutic index drugs. In each study, dexlansoprazole MR 90 mg or placebo was administered once daily for 9 or 11 days in each period. Subjects received a single dose of test substrate in each study period. Pharmacokinetic parameters of the test substrates were estimated using noncompartmental methods. A conclusion of no effect of dexlansoprazole MR on the test substrate was made if the 90% confidence intervals (CIs) for the ratios of the central values for the observed maximum plasma drug concentration (Cmax) and the area under the plasma concentration-time curve (AUC) of test substrate administered with dexlansoprazole MR versus placebo were within 0.80–1.25 based on an analysis of variance model. The potential for a pharmacodynamic interaction was also assessed for warfarin using prothrombin time, measured as the international normalized ratio. Routine safety assessments were conducted in these studies.
Results: Mean Cmax and AUC values were generally similar for each test substrate when administered with multiple once-daily doses of dexlansoprazole MR or placebo. The 90% CIs for the bioavailability of these test substrates administered with dexlansoprazole MR relative to that obtained when the substrates were administered with placebo were within the bioequivalency range of 0.80–1.25, indicating that multiple doses of dexlansoprazole MR had no effect on the pharmacokinetics of these drugs. Additionally, dexlansoprazole MR had no effect on the pharmacodynamics of warfarin. Administration of these drugs with dexlansoprazole MR 90 mg or placebo was well tolerated; the only serious adverse event, which led to a subject’s discontinuation from the study, was considered unrelated to study drugs.
Conclusions: Coadministration of dexlansoprazole MR with diazepam, phenytoin or theophylline did not affect the pharmacokinetics of these drugs, and therefore is unlikely to alter the pharmacokinetic profile of other drugs metabolized by CYP2C19, CYP2C9, CYP1A2 and perhaps CYP3A. Additionally, dexlansoprazole MR coadministered with warfarin did not affect the pharmacokinetics of the warfarin enantiomers and had no effect on the anticoagulant activity of warfarin. Dexlansoprazole MR was well tolerated in these trials of healthy subjects.








Similar content being viewed by others
References
DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol 2005 Jan; 100(1): 190–200
Gerson LB, Triadafilopoulos G. Proton pump inhibitors and their drug interactions: an evidence-based approach. Eur J Gastroenterol Hepatol 2001 May; 13(5): 611–6
Blume H, Donath F, Warnke A, et al. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf 2006; 29(9): 769–84
Reilly JP. Safety profile of the proton-pump inhibitors. Am J Health Syst Pharm 1999 Dec 1; 56(23 Suppl. 4): SI 1–7
Li XQ, Andersson TB, Ahlstrom M, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos 2004 Aug; 32(8): 821–7
Simon W. Pantoprazole: which cytochrome P450 isoenzymes are involved in its biotransformation? [abstract]. Gut 1995; 37: A1177
Humphries TJ, Merritt GJ. Drug interactions with agents used to treat acid-related diseases. Aliment Pharmacol Ther 1999 Aug; 13Suppl. 3: 18–26
Caraco Y, Tateishi T, Wood AJ. Interethnic difference in omeprazole’s inhibition of diazepam metabolism. Clin Pharmacol Ther 1995 Jul; 58(1): 62–72
Andersson T, Cederberg C, Edvardsson G, et al. Effect of omeprazole treatment on diazepam plasma levels in slow versus normal rapid metabolizers of omeprazole. Clin Pharmacol Ther 1990 Jan; 47(1): 79–85
Gugler R, Jensen JC. Omeprazole inhibits oxidative drug metabolism: studies with diazepam and phenytoin in vivo and 7-ethoxycoumarin in vitro. Gastroenterology 1985 Dec; 89(6): 1235–41
Nexium® (esomeprazole magnesium). Full prescribing information. Wilmington (DE): AstraZeneca LP, 2007
Saltiel E, Fask A. Prevalence of potential proton-pump inhibitor drug interactions: a retrospective review of prescriptions in community pharmacies. Clin Ther 1999 Oct; 21(10): 1812–9
McCarthy DM, McLaughlin TP, Griffis DL, et al. Impact of cotherapy with some proton pump inhibitors on medical claims among HMO patients already using other common drugs also cleared by cytochrome P450. Am J Ther 2003 Sep-Oct; 10(5): 330–40
Ramirez FC. Diagnosis and treatment of gastroesophageal reflux disease in the elderly. Cleve Clin J Med 2000 Oct; 67(10): 755–66
van der Meer FJ, Rosendaal FR, Vandenbroucke JP, et al. Bleeding complications in oral anticoagulant therapy: an analysis of risk factors. Arch Intern Med 1993 Jul 12; 153(13): 1557–62
Prevacid® (lansoprazole). Full prescribing information. Lake Forest (IL): TAP Pharmaceutical Products, Inc., 2007
Aciphex® (rabeprazole sodium). Full prescribing information. Tokyo: Eisai Co. Ltd, 2007
Protonix® (pantoprazole sodium). Full prescribing information. Philadelphia (PA): Wyeth Pharmaceuticals, Inc., 2007
Prilosec® (omeprazole). Full prescribing information. Wilmington (DE): AstraZeneca LP, 2007
Zegerid® (omeprazole bicarbonate). Full prescribing information. San Diego (CA): Santarus, Inc., 2006
Zhang W, Wu J, Atkinson S. Pharmacokinetics, pharmaco-dynamics, and safety evaluation of a single and multiple 60 mg, 90 mg, and 120 mg oral doses of modified-release TAK-390 (TAK-390MR) and 30 mg oral doses of lansoprazole in healthy subjects [abstract]. Gastroenterology 2007; 132Suppl. 52: A87
Liu KH, Kim MJ, Jung WM, et al. Lansoprazole enantiomer activates human liver microsomal CYP2C9 catalytic activity in a stereospecific and substrate-specific manner. Drug Metab Dispos 2005 Feb; 33(2): 209–13
Curi-Pedrosa R, Daujat M, Pichard L, et al. Omeprazole and lansoprazole are mixed inducers of CYP1A and CYP3A in human hepatocytes in primary culture. J Pharmacol Exp Ther 1994 Apr; 269(1): 384–92
Granneman GR, Karol MD, Locke CS, et al. Pharmacokinetic interaction between lansoprazole and theophylline. Ther Drug Monit 1995 Oct; 17(5): 460–4
Inomata S, Nagashima A, Itagaki F, et al. CYP2C19 genotype affects diazepam pharmacokinetics and emergence from general anesthesia. Clin Pharmacol Ther 2005 Dec; 78(6): 647–55
Andersson T, Rohss K, Bredberg E, et al. Pharmacokinetics and pharmacodynamics of esomeprazole, the S-isomer of omeprazole. Aliment Pharmacol Ther 2001 Oct; 15(10): 1563–9
Lee SY, Lee ST, Kim JW. Contributions of CYP2C9/CYP2C19 genotypes and drug interaction to the phenytoin treatment in the Korean epileptic patients in the clinical setting. J Biochem Mol Biol 2007 May 31; 40(3): 448–52
Horsmans Y, Van den Berge V, Bouckaert A, et al. Phenytoin hydroxylation in a healthy Caucasian population: bimodal distribution of hydroxyphenytoin urinary excretion. Pharmacol Toxicol 1997; 8(6): 276–9
Sarkar MA, Hunt C, Guzelian PS, et al. Characterization of human liver cytochromes P-450 involved in theophylline metabolism. Drug Metab Dispos 1992 Jan-Feb; 20(1): 31–7
Priskorn M, Sidhu JS, Larsen F, et al. Investigation of multiple dose citalopram on the pharmacokinetics and pharmacodynamics of racemic warfarin. Br J Clin Pharmacol 1997; 44: 199–202
Dingemanse J, Meyerhoff C, Schadrack J. Effect of the catechol-O-methyl transferase inhibitor entacapone on the steadystate pharmacokinetics and pharmacodynamics of warfarin. Br J Clin Pharmacol 2002 May; 53(5): 485–91
Reynolds KK, Valdes R, Hartung BR, et al. Individualizing warfarin therapy. Personalized Med 2007; 4(1): 11–31
Lefebvre RA, Flouvat B, Karolac-Tamisier S, et al. Influence of lansoprazole treatment on diazepam plasma concentration. Clin Pharmacol Ther 1992; 52(5): 458–63
Prichard PJ, Walt RP, Kitchingman GK, et al. Oral phenytoin pharmacokinetics during omeprazole therapy. Br J Clin Pharmacol 1987; 24(4): 543–5
Dilger K, Zheng Z, Klotz U. Lack of drug interaction between omeprazole, lansoprazole, pantoprazole and theophylline. Br J Clin Pharmacol 1999; 48(3): 438–44
Study No. (C03-057). Lake Forest (IL): Takeda Global Research & Development Center, Inc., 2004. (Data on file)
Juurlink DN, Mamdani M, Kopp A, et al. Drug-drug interactions among elderly patients hospitalized for drug toxicity. JAMA 2003 Apr 2; 289(13): 1652–8
Andersson T, Hassan-Alin M, Hasselgren G, et al. Drug interaction studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet 2001; 40(7): 523–37
Sutfin T, Balmer K, Bostrom H, et al. Stereoselective interaction of omeprazole with warfarin in healthy men. Ther Drug Monit 1989; 11(2): 176–84
Unge P, Svedberg LE, Nordgren A, et al. A study of the interaction of omeprazole and warfarin in anticoagulated patients. Br J Clin Pharmacol 1992 Dec; 34(6): 509–12
Acknowledgements
All authors are employees of Takeda Global Research and Development Center, Inc., Deerfield, IL, USA (TAP Pharmaceutical Products Inc., Lake Forest, IL, USA, is now a part of Takeda Global Research & Development Center, Inc.). All authors participated in the development and writing of this article and approved the final manuscript for publication. The authors take full responsibility for the content of the article and wish to acknowledge the assistance in manuscript preparation provided by Eileen Gallagher of Complete Healthcare Communications, Inc., Chadds Ford, PA, USA, and funded by Takeda Global Research and Development Center, Inc. We also thank our colleague Michael Mayer for his editorial review and comments. All studies (T-P105-132 [warfarin], T-P105-133 [phenytoin], T-P105-134 [diazepam] and T-P105-139 [theophylline]) were sponsored by Takeda Global Research & Development Center, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vakily, M., Lee, R.D., Wu, J. et al. Drug Interaction Studies with Dexlansoprazole Modified Release (TAK-390MR), a Proton Pump Inhibitor with a Dual Delayed-Release Formulation. Clin. Drug Investig. 29, 35–50 (2009). https://doi.org/10.2165/0044011-200929010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/0044011-200929010-00004