Abstract
Background and Objective: Filgrastim is a human granulocyte-colony stimulating factor (G-CSF). The biological activity of filgrastim is identical to that of endogenous G-CSF. It controls neutrophil production within the bone marrow by stimulating the proliferation, differentiation and survival of myeloid progenitor cells and some end-cell function activation. The purpose of this work is to propose a target-mediated drug disposition pharmacokinetic model of filgrastim.
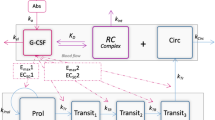
Methods: A mechanism-based population pharmacokinetic model was developed to account for receptormediated endocytosis as a mechanism for nonlinear disposition of G-CSF. Time profiles of serum filgrastim concentrations following subcutaneous doses of 2.5, 5 and 10 μg/kg and intravenous infusion of 5 μg/kg over 0.5 hour were studied. The pharmacokinetic model included first-order elimination from the serum, receptor binding, turnover of free receptors and internalization of drug-receptor complexes. The proposed targetmediated drug disposition models served as a tool to study drug absorption and the impact of receptor binding on filgrastim clearance.
Results: Filgrastim was found to exhibit parallel absorption with first- and zero-order kinetics and bioavailability of 69.1%. The majority of the drug (58.6%) was absorbed by zero-order processes, presumably through the lymphatic system. The equilibrium dissociation constant (Kd) was estimated as 16.38 pM.
Conclusion: The proposed model predicts that clearance is initially mostly governed by the binding of filgrastim to G-CSF receptors. Subsequently, the clearance slows down because of the saturation of binding sites, and occurs mostly via the linear (renal) pathway. Finally, for G-CSF concentrations lower than the Kd, target-mediated clearance dominates. The presented receptor-mediated model adequately describes filgrastim serum concentrations and quantifies the role of receptor binding in G-CSF clearance.







Similar content being viewed by others
References
Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood 1991 Dec 1; 78(11): 2791–808
Nicola NA, Metcalf D. Binding of 125I-labeled granulocyte colony-stimulating factor to normal murine hemopoietic cells. J Cell Physiol 1985 Aug; 124(2): 313–21
Anderlini P, Champlin RE. Biologic and molecular effects of granulocyte colony-stimulating factor in normal individuals: recent findings and current challenges. Blood 2008 Feb 15; 111(4): 1767–72
Robinson CR. Reduce, reuse, and recycle. Nature Biotechnol 2002 Sep; 20(9): 879–80
Morstyn G, Dexter TM. Filgrastim (r-metHuG-CSF) in clinical practice. New York: Marcel Dekker, 1994
Price TH, Chatta GS, Dale DC. Effect of recombinant granulocyte colonystimulating factor on neutrophil kinetics in normal young and elderly humans. Blood 1996 Jul 1; 88(1): 335–40
Sung L, Dror Y. Clinical applications of granulocyte-colony stimulating factor. Front Biosci 2007; 12: 1988–2002
Borleffs JC, Bosschaert M, Vrehen HM, et al. Effect of escalating doses of recombinant human granulocyte colony-stimulating factor (filgrastim) on circulating neutrophils in healthy subjects. Clin Ther 1998 Jul–Aug; 20(4): 722–36
Wang B, Ludden TM, Cheung EN, et al. Population pharmacokineticpharmacodynamic modeling of filgrastim (r-metHuG-CSF) in healthy volunteers. J Pharmacokinet Pharmacodyn 2001 Aug; 28(4): 321–42
Kuwabara T, Uchimura T, Kobayashi H, et al. Receptor-mediated clearance of G-CSF derivative nartograstim in bone marrow of rats. Am J Physiol 1995 Jul; 269(1 Pt 1): E1–9
Layton JE, Hockman H, Sheridan WP, et al. Evidence for a novel in vivo control mechanism of granulopoiesis: mature cell-related control of a regulatory growth factor. Blood 1989 Sep; 74(4): 1303–7
Levy G. Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 1994 Sep; 56(3): 248–52
Mager DE, Jusko WJ. General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 2001 Dec; 28(6): 507–32
Joshi A, Bauer R, Kuebler P, et al. An overview of the pharmacokinetics and pharmacodynamics of efalizumab: a monoclonal antibody approved for use in psoriasis. J Clin Pharmacol 2006 Jan; 46(1): 10–20
Ng CM, Stefanich E, Anand BS, et al. Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm Res 2006 Jan; 23(1): 95–103
Krzyzanski W, Wyska E. Pharmacokinetics and pharmacodynamics of erythropoietin receptor in healthy volunteers. Naunyn Schmiedebergs Arch Pharmacol 2008 Jun; 377(4–6): 637–45
Woo S, Krzyzanski W, Jusko WJ. Target-mediated pharmacokinetic and pharmacodynamic model of recombinant human erythropoietin (rHuEPO). J Pharmacokinet Pharmacodyn 2007 Dec; 34(6): 849–68
Kawakami M, Tsutsumi H, Kumakawa T, et al. Levels of serum granulocyte colony-stimulating factor in patients with infections. Blood 1990 Nov 15; 76(10): 1962–4
Roskos LK, Lum P, Lockbaum P, et al. Pharmacokinetic/pharmacodynamic modeling of pegfilgrastim in healthy subjects. J Clin Pharmacol 2006 Jul; 46(7): 747–57
Hayashi N, Kinoshita H, Yukawa E, et al. Pharmacokinetic and pharmacodynamic analysis of subcutaneous recombinant human granulocyte colony stimulating factor (lenograstim) administration. J Clin Pharmacol 1999 Jun; 39(6): 583–92
Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed 1999 Apr; 59(1): 19–29
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res 1993 Jul; 10(7): 1093–5
Skubitz KM. Neutrophilic leukocytes. In: Wintrobe MM, Greer J, editors. Wintrobe’s clinical hematology. 11th ed. Vol. 1. Philadelphia (PA): Lippincott Williams & Wilkins, 2004; 170–214
Avalos BR, Gasson JC, Hedvat C, et al. Human granulocyte colonystimulating factor: biologic activities and receptor characterization on hematopoietic cells and small cell lung cancer cell lines. Blood 1990 Feb 15; 75(4): 851–7
Sarkar CA, Lowenhaupt K, Wang PJ, et al. Parsing the effects of binding, signaling, and trafficking on the mitogenic potencies of granulocyte colony-stimulating factor analogues. Biotechnol Prog 2003 May–Jun; 19(3): 955–64
Sarkar CA, Lowenhaupt K, Horan T, et al. Rational cytokine design for increased lifetime and enhanced potency using pH-activated “histidine switching”. Nature Biotechnol 2002 Sep; 20(9): 908–13
Acknowledgements
This study was sponsored by Novartis Pharmaceuticals AG (Basel, Switzerland). Etienne Pigeolet and Philip Lowe are employees of Novartis. Philip Lowe owns shares in Novartis. Wojciech Krzyzanski has received grants from Novartis. Paweł Wiczling, Frank Lüdicke and Sigrid Balser have no conflicts of interest that are directly relevant to the contents of this study.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The target-mediated disposition model of G-CSF was initially written in molar concentrations as follows (equations 15–17):
where the square brackets denote molar concentrations and MG-CSF is the molar mass of G-CSF. Multiplying the above equations by MG-CSF and Vd led to equations 18–20:
where As, AR and ARC denoted the amounts of G-CSF, its receptor and drug-receptor complex in MG-CSF units, and kon = k′on on=MG–CSF
Rights and permissions
About this article
Cite this article
Wiczling, P., Lowe, P., Pigeolet, E. et al. Population Pharmacokinetic Modelling of Filgrastim in Healthy Adults following Intravenous and Subcutaneous Administrations. Clin Pharmacokinet 48, 817–826 (2009). https://doi.org/10.2165/11318090-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11318090-000000000-00000