Abstract
Background and Objectives: An approach was recently proposed for quantitative predictions of cytochrome P450 (CYP) 3A4-mediated drug-drug interactions. This approach relies solely on in vivo data. It is based on two characteristic parameters: the contribution ratio (CR; i.e. the fraction of victim drug clearance due to metabolism by a specific CYP) and the inhibition ratio (IR) of the inhibitor. Knowledge of these parameters allows forecasting of the ratio between the area under the plasma concentration-time curve (AUC) of the victim drug when the inhibitor is co-administered and the AUC of the victim drug administered alone. The goals of our study were to extend this method to CYP2D6-mediated interactions, to validate it, and to forecast the magnitude of a large number of interactions that have not been studied so far.
Methods: A three-step approach was pursued. First, initial estimates of CRs and IRs were obtained by several methods, using data from the literature. Second, an external validation of these initial estimates was carried out, by comparing the predicted AUC ratios with the observed values. Third, refined estimates of CRs and IRs were obtained by orthogonal regression in a Bayesian framework.
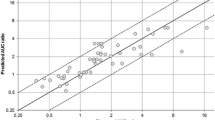
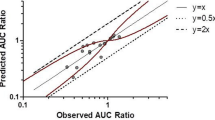
Results: Thirty-nine AUC ratios were available for external validation. The mean prediction error of the ratios was 0.31, while the mean prediction absolute error was 1.14. Seventy AUC ratios were available for the global analysis. Final estimates of CRs and IRs were obtained for 39 substrates and 11 inhibitors, respectively. The mean prediction error of the AUC ratios was 0.04, while the mean prediction absolute error was 0.51.
Conclusions: Predictive distributions for 615 possible interactions were obtained, giving detailed information on some drugs or inhibitors that have been poorly studied so far, such as metoclopramide, bupropion and terbinafine.








Similar content being viewed by others
References
Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet 2009; 48(11): 689–723
Campbell TJ, Williams KM. Therapeutic drug monitoring: antiarrhythmic drugs. Br J Clin Pharmacol 2001; 52 Suppl. 1: 21S–34S
Gibbs JP, Hyland R, Youdim K. Minimizing polymorphic metabolism in drug discovery: evaluation of the utility of in vitro methods for predicting pharmacokinetic consequences associated with CYP2D6 metabolism. Drug Metab Dispos 2006 Sep; 34(9): 1516–22
Waade RB, Christensen H, Rudberg I. Influence of comedication on serum concentrations of aripiprazole and dehydroaripiprazole. Ther Drug Monit 2009 Apr; 31(2): 233–8
Venkatakrishnan K, Obach RS. In vitro-in vivo extrapolation of CYP2D6 inactivation by paroxetine: prediction of nonstationary pharmacokinetics and drug interaction magnitude. Drug Metab Dispos 2005 Mar; 33(6): 845–52
Giessmann T, Modess C, Hecker U, et al. CYP2D6 genotype and induction of intestinal drug transporters by rifampin predict presystemic clearance of carvedilol in healthy subjects. Clin Pharmacol Ther 2004 Mar; 75(3): 213–22
Yasuda US, Zannikos P, Andrea E. The roles of CYP2D6 and stereoselectivity in the clinical pharmacokinetics of chlorpheniramine. Br J Clin Pharmacol 2002; 53: 519–25
Vree TB, Verwey-van Wissen CP. Pharmacokinetics and metabolism of codeine in humans. Biopharm Drug Dispos 1992 Aug; 13(6): 445–60
Caraco Y, Sheller J, Wood AJ. Pharmacogenetic determination of the effects of codeine and prediction of drug interactions. J Pharmacol Exp Ther 1996 Sep; 278(3): 1165–74
Skinner MH, Kuan HY, Pan A. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther 2003 Mar; 73(3): 170–7
Birgersdotter UW, Wong W, Turgeon J. Stereoselective genetically-determined interaction between chronic flecainide and quinidine in patients with arrhythmias. Br J Clin Pharmacol 1992; 33: 275–80
Albers LJ, Reist C, Helmeste D. Paroxetine shifts imipramine metabolism. Psychiatry Res 1996 Jan; 59(3): 189–96
Vlase L, Leucuta A, Farcau D, et al. Pharmacokinetic interaction between fluoxetine and metoclopramide in healthy volunteers. Biopharm Drug Dispos 2006 Sep; 27(6): 285–9
Kirchheiner J, Heesch C, Bauer S, et al. Impact of the ultrarapid metabolizer genotype of cytochrome P450 2D6 on metoprolol pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2004 Oct; 76(4): 302–12
Abolfathi Z, Fiset C, Gilbert M. Role of polymorphic debrisoquin 4-hydroxylase activity in the stereoselective disposition of mexiletine in humans. J Pharmacol Exp Ther 1993 Mar; 266(3): 1196–201
Yasui N, Tybring G, Otani K. Effects of thioridazine, an inhibitor of CYP2D6, on the steady-state plasma concentrations of the enantiomers of mianserin and its active metabolite, desmethylmianserin, in depressed Japanese patients. Pharmacogenetics 1997 Oct; 7(5): 369–74
Ruwe FJ, Smulders RA, Kleijn HJ. Mirtazapine and paroxetine: a drug-drug interaction study in healthy subjects. Hum Psychopharmacol 2001 Aug; 16(6): 449–59
Dalén P, Dahl ML, Bernal Ruiz ML, et al. 10-Hydroxylation of nortriptyline in White persons with 0, 1, 2, 3, and 13 functional CYP2D6 genes. Clin Pharmacol Ther 1998 Apr; 63(4): 444–52
Molden E, Garcia BH, Braathe P. Co-prescription of cytochrome P450 2D6/3A4 inhibitor-substrate pairs in clinical practice: a retrospective analysis of data from Norwegian primary pharmacies. Eur J Clin Pharmacol 2005; 61: 119–25
Scordo MG, Spina` E, Facciola` G, et al. Cytochrome P450 2D6 genotype and steady state plasma levels of risperidone and 9-hydroxyrisperidone. Psycho-pharmacology (Berl) 1999 Dec; 147(3): 300–5
Berecz R, de la Rubia A, Dorado P, et al. Thioridazine steady-state plasma concentrations are influenced by tobacco smoking and CYP2D6, but not by the CYP2C9 genotype. Eur J Clin Pharmacol 2003 May; 59(1): 45–50
Pedersen RS, Damkier P, Bøsen K. Enantioselective pharmacokinetics of tramadol in CYP2D6 extensive and poor metabolizers. Eur J Clin Pharmacol 2006 Jul; 62(7): 513–21
Kirchheiner J, Müller G, Meineke I, et al. Effects of polymorphisms in CYP2D6, CYP2C9, and CYP2C19 on trimipramine pharmacokinetics. J Clin Psychopharmacol 2003 Oct; 23(5): 459–66
Eap CB, Lessard E, Baumann P. Role of CYP2D6 in the stereoselective disposition of venlafaxine in humans. Pharmacogenetics 2003; 13(1): 39–47
Kaiser R, Sezer O, Papies A, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes. J Clin Oncol 2002 Jun 15; 20(12): 2805–11
Jaanson P, Marandi T, Kiivet RA, et al. Maintenance therapy with zuclopenthixol decanoate: associations between plasma concentrations, neurological side effects and CYP2D6 genotype. Psychopharmacology (Berl) 2002 Jun; 162(1): 67–73
Reese MJ, Wurm RM, Muir KT. An in vitro mechanistic study to elucidate the desipramine/bupropion clinical drug-drug interaction. Drug Metab Dispos 2008; 36(7): 1198–201
Hamelin BA, Bouayad A, Méthot J, et al. Significant interaction between the nonprescription antihistamine diphenhydramine and the CYP2D6 substrate metoprolol in healthy men with high or low CYP2D6 activity. Clin Pharmacol Ther 2000 May; 67(5): 466–77
Preskorn SH, Greenblatt DJ, Flockhart D. Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol 2007 Feb; 27(1): 28–34
Preskorn SH, Alderman J, Chung M. Pharmacokinetics of desipramine coadministered with sertraline or fluoxetine. J Clin Psychopharmacol 1994 Apr; 14(2): 90–8
Bergstrom RF, Peyton AL, Lemberger L. Quantification and mechanism of the fluoxetine and tricyclic antidepressant interaction. Clin Pharmacol Ther 1992; 51(3): 239–48
Kallio J, Huupponen R, Seppälä M, et al. The effects of beta-adrenoceptor antagonists and levomepromazine on the metabolic ratio of debrisoquine. Br J Clin Pharmacol 1990; 30: 638–43
Stout SM, Nielsen J, Bleske BE, et al. The impact of paroxetine coadministration on stereospecific carvedilol pharmacokinetics. J Cardiovasc Pharmacol Ther 2010 Dec; 15(4): 373–9
Kowey PR, Kirsten EB, Fu CH. Interaction between propranolol and propafenone in healthy volunteers. J Clin Pharmacol 1989 Jun; 29(6): 512–7
Capon DA, Bochner F, Kerry N. The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans. Clin Pharmacol Ther 1996 Sep; 60(3): 295–307
Solai LK, Mulsant BH, Pollock BG, et al. Effect of sertraline on plasma nor-triptyline levels in depressed elderly. J Clin Psychiatry 1997 Oct; 58(10): 440–3
Madani S, Barilla D, Cramer J. Effect of terbinafine on the pharmacokinetics and pharmacodynamics of desipramine in healthy volunteers identified as cytochrome P450 2D6 (CYP2D6) extensive metabolizers. J Clin Pharmacol 2002 Nov;42(11): 1211–8
Nakagami T, Yasui-Furukori N, Saito M, et al. Thioridazine inhibits risperidone metabolism: a clinically relevant drug interaction. J Clin Psychopharmacol 2005 Feb; 25(1): 89–91
el-Yazigi A, Chaleby K, Gad A, et al. Steady-state kinetics of fluoxetine and amitriptyline in patients treated with a combination of these drugs as compared with those treated with amitriptyline alone. J Clin Pharmacol 1995 Jan; 35(1): 17–21
Castberg I, Helle J, Aamo T. Prolonged pharmacokinetic drug interaction between terbinafine and amitriptyline. Ther Drug Monit 2005 Oct; 27(5): 680–2
Yasuda SU, Zannikos P, Young AE, et al. The roles of CYP2D6 and stereoselectivity in the clinical pharmacokinetics of chlorpheniramine. Br J Clin Pharmacol 2002 May; 53(5): 519–25
Vevelstad M, Pettersen S, Tallaksen C. O-demethylation of codeine to morphine inhibited by low-dose levomepromazine. Eur J Clin Pharmacol 2009 Aug; 65(8): 795–801
Patroneva A, Connolly SM, Fatato P. An assessment of drug-drug interactions: the effect of desvenlafaxine and duloxetine on the pharmacokinetic of the CYP2D6 probe desipramine in healthy subjects. Drug Metab Dispos 2008 Sep; 36(12): 2484–91
Skinner MH, Kuan HY, Pan A, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther 2003 Mar; 73(3): 170–7
Kurtz DL, Bergstrom RF, Goldberg MJ, et al. The effect of sertraline on the pharmacokinetics of desipramine and imipramine. Clin Pharmacol Ther 1997 Aug; 62(2): 145–56
Brøsen K, Gram LF. Quinidine inhibits the 2-hydroxylation of imipramine and desipramine but not the demethylation of imipramine. Eur J Clin Pharmacol 1989; 37(2): 155–60
Johnson JA, Burlew BS. Metoprolol metabolism via cytochrome P4502D6 in ethnic populations. Drug Metab Dispos 1996 Mar; 24(3): 350–5
Sharma A, Pibarot P, Pilote S, et al. Toward optimal treatment in women: the effect of sex on metoprolol-diphenhydramine interaction. J Clin Pharmacol 2010 Feb; 50(2): 214–25
Laine K, Tybring G, Härtter S, et al. Inhibition of cytochrome P4502D6 activity with paroxetine normalizes the ultrarapid metabolizer phenotype as measured by nortriptyline pharmacokinetics and the debrisoquin test. Clin Pharmacol Ther 2001 Oct; 70(4): 327–35
Yasui-Furukori N, Saito M, Inoue Y. Terbinafine increases the plasma concentration of paroxetine after a single oral administration of paroxetine in healthy subjects. Eur J Clin Pharmacol 2007 Jan; 63(1): 51–6
Funck-Brentano C, Kroemer HK, Pavlou H. Genetically-determined interaction between propafenone and low dose quinidine: role of active metabolites in modulating net drug effect. Br J Clin Pharmacol 1989 Apr; 27(4): 435–44
Cai WM, Chen B, Zhou Y. Fluoxetine impairs the CYP2D6-mediated metabolism of propafenone enantiomers in healthy Chinese volunteers. Clin Pharmacol Ther 1999 Nov; 66(5): 516–21
Spinà E, Avenoso A, Scordo MG. Inhibition of risperidone metabolism by fluoxetine in patients with schizophrenia: a clinically relevant pharmacokinetic drug interaction. J Clin Psychopharmacol 2002 Aug; 22(4): 419–23
Yoshimura R, Shinkai K, Kakihara S, et al. Little effects of low dosage of levomepromazine on plasma risperidone levels. Pharmacopsychiatry 2005 Mar; 38(2): 98–100
Saito M, Yasui-Furukori N, Nakagami T. Dose-dependent interaction of paroxetine with risperidone in schizophrenic patients. J Clin Psychopharmacol 2005 Dec; 25(6): 527–32
Brynne N, Svanstrom C, Aberg-Wistedt A. Fluoxetine inhibits the metabolism of tolterodine: pharmacokinetic implications and proposed clinical relevance. Br J Clin Pharmacol 1999; 48: 553–63
Laugesen S, Enggaard TP, Pedersen RS, et al. Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther 2005 Apr; 77(4): 312–23
Eap CB, Laurian S, Souche A. Influence of quinidine on the pharmacokinetics of trimipramine and on its effect on the waking EEG of healthy volunteers: a pilot study on two subjects. Neuropsychobiol 1992; 25(4): 214–20
Kennedy SH, McCann SM, Masellis M. Combining bupropion SR with venlafaxine, paroxetine, or fluoxetine: a preliminary report on pharmacokinetic, therapeutic, and sexual dysfunction effects. J Clin Psychiatry 2002 Mar; 63(3): 181–6
Lessard E, Yessine MA, Hamelin BA. Diphenhydramine alters the disposition of venlafaxine through inhibition of CYP2D6 activity in humans. J Clin Psychopharmacol 2001 Apr; 21(2): 175–84
Eap CB, Lessard E, Baumann P, et al. Role of CYP2D6 in the stereoselective disposition of venlafaxine in humans. Pharmacogenetics 2003 Jan; 13(1): 39–47
Ito K, Brown HS, Houston JB. Database analyses for the prediction of in vivo drug-drug interactions from in vitro data. Br J Clin Pharmacol 2004 Apr; 57(4): 473–86
Bertelsen KM, Venkatakrishnan K, von Moltke LL, et al. Apparent mechanism-based inhibition of human CYP2D6 in vitro by paroxetine: comparison with fluoxetine and quinidine. Drug Metab Dispos 2003 Mar; 31(3): 289–93
Ohno Y, Hisaka A, Suzuki H. General framework for the quantitative prediction of CYP3A4-mediated oral drug interactions based on the AUC increase by coadministration of standard drugs. Clin Pharmacokinet 2007; 46(8): 681–96
Ohno Y, Hisaka A, Ueno M, et al. General framework for the prediction of oral drug interactions caused by CYP3A4 induction from in vivo information. Clin Pharmacokinet 2008; 47(10): 669–80
Thelen K, Dressman JB. Cytochrome P450-mediated metabolism in the human gut wall. J Pharm Pharmacol 2009 May; 61(5): 541–58
Avenoso A, Spinà E, Campo G. Interaction between fluoxetine and haloperidol: pharmacokinetic and clinical implications. Pharmacol Res 1997 Apr; 35(4): 335–9
Eap CB, Bertschy G, Powell K, et al. Fluvoxamine and fluoxetine do not interact in the same way with the metabolism of the enantiomers of metha-done. J Clin Psychopharmacol 1997 Apr; 17(2): 113–7
Amoah AG, Gould BJ, Parke DV. Single-dose pharmacokinetics of perhexiline administered orally to humans. J Chromatogr 1984 Feb 10; 305(2): 401–9
Jordan VC. New insights into the metabolism of tamoxifen and its role in the treatment and prevention of breast cancer. Steroids 2007 Nov; 72(13): 829–42
Hamelin BA, Bouayad A, Drolet B, et al. In vitro characterization of cytochrome P450 2D6 inhibition by classic histamine H1 receptor antagonists. Drug Metab Dispos 1998 Jun; 26(6): 536–9
Sauer JM, Long AJ, Ring B, et al. Atomoxetine hydrochloride: clinical drug-drug interaction prediction and outcome. J Pharmacol Exp Ther 2004 Feb; 308(2): 410–8
Zhang W, Ramamoorthy Y, Tyndale RF, et al. Interaction of buprenorphine and its metabolite norbuprenorphine with cytochromes p450 in vitro. Drug Metab Dispos 2003 Jun; 31(6): 768–72
Wennerholm A, Nordmark A, Pihlsgård M, et al. Amodiaquine, its desethy-lated metabolite, or both, inhibit the metabolism of debrisoquine (CYP2D6) and losartan (CYP2C9) in vivo. Eur J Clin Pharmacol 2006 Jul; 62(7): 539–46
Simooya O, Sijumbil G, Lennard MS. Halofantrine and chloroquine inhibit CYP2D6 activity in healthy Zambians. Br J Clin Pharmacol 1998; 45: 315–7
von Moltke LL, Greenblatt DJ, Grassi JM, et al. Protease inhibitors as inhibitors of human cytochromes P450: high risk associated with ritonavir. J Clin Pharmacol 1998 Feb; 38(2): 106–11
Fukumoto K, Kobayashi T, Tachibana K. Effect of amiodarone on the serum concentration/dose ratio of metoprolol in patients with cardiac arrhythmia. Drug Metab Pharmacokinet 2006; 21(6): 501–5
Congdon PC. Bayesian statistical modelling. 2nd ed. New York: John Wiley & Sons, Inc., 2006
Spiegelhalter D, Thomas A, Best N, et al. WinBugs 1.4 user manual. Cambridge: Institute of Public Health, 2004
Fromm MF, Kim RB, Stein CM, et al. Inhibition of P-glycoprotein-mediated drug transport: a unifying mechanism to explain the interaction between di-goxin and quinidine. Circulation 1999 Feb 2; 99(4): 552–7
Acknowledgements
No sources of funding were used to conduct this study or prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The orthogonal regression was based on the following approach:
•CRs and IRs are the initial values found in step 1 of the analysis.
•X’s and Y’s are the logit-transformed initial values.
•CRTs and IRTs are the ‘observed’ logit-transformed initial values.
•CRZs and IRZs are the refined estimates.
•∼ N(μ, τ) means ‘distributed as normal distribution, with a mean of μ and precision of τ’. The precision is the inverse of the variance.
•∼ G(r, μ) means ‘distributed as gamma distribution, with a mean of r/μ and variance of r/μ*’.
•i and j are the indexes of the substrate and the inhibitor, respectively.
•preds are the predicted AUC ratios for each (CR, IR) couple.
•AUC ratios are the observed values, if any, for each (CR, IR) couple.
For each j:

For each i:

Rights and permissions
About this article
Cite this article
Tod, M., Goutelle, S., Clavel-Grabit, F. et al. Quantitative Prediction of Cytochrome P450 (CYP) 2D6-Mediated Drug Interactions. Clin Pharmacokinet 50, 519–530 (2011). https://doi.org/10.2165/11592620-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11592620-000000000-00000