Abstract
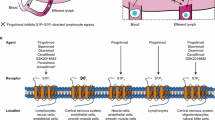
Fingolimod (FTY720), a sphingosine 1-phosphate receptor modulator, is the first in a new class of therapeutic compounds and is the first oral therapy approved for the treatment of relapsing forms of multiple sclerosis (MS). Fingolimod is a structural analogue of endogenous sphingosine and undergoes phosphorylation to produce fingolimod phosphate, the active moiety. Fingolimod targets MS via effects on the immune system, and evidence from animal models indicates that it may also have actions in the central nervous system. In phase III studies in patients with relapsing-remitting MS, fingolimod has demonstrated efficacy superior to that of an approved first-line therapy, intramuscular interferon-β-1a, as well as placebo, with benefits extending across clinical and magnetic resonance imaging measures.
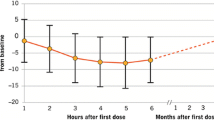
The pharmacokinetic profiles of fingolimod and fingolimod phosphate have been extensively investigated in studies in healthy volunteers, renal transplant recipients (the indication for which fingolimod was initially under clinical development, but the development was subsequently discontinued) and MS patients. Results from these studies have demonstrated that fingolimod is efficiently absorbed, with an oral bioavailability of >90%, and its absorption is unaffected by dietary intake, therefore it can be taken without regard to meals. Fingolimod and fingolimod phosphate have a half-life of 6–9 days, and steady-state pharmacokinetics are reached after 1–2 months of daily dosing. The long half-life of fingolimod, together with its slow absorption, means that fingolimod has a flat concentration profile over time with once-daily dosing. Fingolimod and fingolimod phosphate show dose-proportional exposure in single- and multiple-dose studies over a range of 0.125–5 mg; hence, there is a predictable relationship between dose and systemic exposure. Furthermore, fingolimod and fingolimod phosphate exhibit low to moderate intersubject pharmacokinetic variability.
Fingolimod is extensively metabolized, with biotransformation occurring via three main pathways: (i) reversible phosphorylation to fingolimod phosphate; (ii) hydroxylation and oxidation to yield a series of inactive carboxylic acid metabolites; and (iii) formation of non-polar ceramides. Fingolimod is largely cleared through metabolism by cytochrome P450 (CYP) 4F2. Since few drugs are metabolized by CYP4F2, fingolimod would be expected to have a relatively low potential for drug-drug interactions. This is supported by data from in vitro studies indicating that fingolimod and fingolimod phosphate have little or no capacity to inhibit and no capacity to induce other major drug-metabolizing CYP enzymes at therapeutically relevant steady-state blood concentrations. Population pharmacokinetic evaluations indicate that CYP3A inhibitors and CYP3A inducers have no effect or only a weak effect on the pharmacokinetics of fingolimod and fingolimod phosphate. However, blood concentrations of fingolimod and fingolimod phosphate are increased moderately when fingolimod is coadministered with ketoconazole, an inhibitor of CYP4F2. The pharmacokinetics of fingolimod are unaffected by renal impairment or mild-to-moderate hepatic impairment. However, exposure to fingolimod is increased in patients with severe hepatic impairment. No clinically relevant effects of age, sex or ethnicity on the pharmacokinetics of fingolimod have been observed.
Fingolimod is thus a promising new therapy for eligible patients with MS, with a predictable pharmacokinetic profile that allows effective once-daily oral dosing.










Similar content being viewed by others
References
Brinkmann V, Davis MD, Heise CE, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem 2002; 277(24): 21453–7
Adachi K, Chiba K. FTY720 story: its discovery and the following accelerated development of sphingosine 1-phosphate receptor agonists as immuno-modulators based on reverse pharmacology. Perspect Medicin Chem 2007; 1: 11–23
Gilenya™ (fingolimod hydrochloride tablets): US prescribing information [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022527s002lbl.pdf [Accessed 2011 Nov 9]
European Medicines Agency. Gilenya (fingolimod): summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002202/WC500104528.pdf [Accessed 2011 Mar 30]
Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol 2010; 33(2): 91–101
Brinkmann V. FTY720 (fingolimod) in multiple sclerosis: therapeutic effects in the immune and the central nervous system. Br J Pharmacol 2009; 158(5): 1173–82
Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362(5): 402–15
Kappos L, Radue EW, O’Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010; 362(5): 387–401
Center for Drug Evaluation and Research [CDER]. Application number: 22-527. Clinical pharmacology and biopharmaceutics review(s) [online]. Available from URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2010/022527Orig1s000clinpharmr.pdf [Accessed 2011 Nov 9]
Kovarik JM, Schmouder R, Barilla D, et al. Single-dose FTY720 pharmacokinetics, food effect, and pharmacological responses in healthy subjects. Br J Clin Pharmacol 2004; 57(5): 586–91
Zollinger M, Gschwind HP, Jin Y, et al. Absorption and disposition of the sphingosine 1-phosphate receptor modulator fingolimod (FTY720) in healthy volunteers: a case of xenobiotic biotransformation following endogenous metabolic pathways. Drug Metab Dispos 2011; 39(2): 199–207
Jin Y, Zollinger M, Borell H, et al. CYP4F enzymes are responsible for the elimination of fingolimod (FTY720), a novel treatment of relapsing multiple sclerosis. Drug Metab Dispos 2011; 39(2): 191–8
Kovarik JM, Slade A, Voss B, et al. Ethnic sensitivity study of fingolimod in White and Asian subjects. Int J Clin Pharmacol Ther 2007; 45(2): 98–109
Kovarik JM, Schmouder RL, Serra D, et al. FTY720 pharmacokinetics in mild to moderate hepatic impairment. J Clin Pharmacol 2005; 45(4): 446–52
Kovarik JM, Schmouder RL, Hartmann S, et al. Fingolimod (FTY720) in severe hepatic impairment: pharmacokinetics and relationship to markers of liver function. J Clin Pharmacol 2006; 46(2): 149–56
Zimmerlin AG, Patten CJ. Role of CYP4F in the metabolic clearance of FTY720: prediction of low drug to drug interaction potential [abstract]. Transplantation 2000; 69 Suppl.: S191
Benet LZ, Hoener BA. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther 2002; 71(3): 115–21
Budde K, Schmouder RL, Brunkhorst R, et al. First human trial of FTY720, a novel immunomodulator, in stable renal transplant patients. J Am Soc Nephrol 2002; 13(4): 1073–83
Kahan BD, Karlix JL, Ferguson RM, et al. Pharmacodynamics, pharmacokinetics, and safety of multiple doses of FTY720 in stable renal transplant patients: a multicenter, randomized, placebo-controlled, phase I study. Transplantation 2003; 76(7): 1079–84
Kovarik JM, Schmouder R, Barilla D, et al. Multiple-dose FTY720: tolerability, pharmacokinetics, and lymphocyte responses in healthy subjects. J Clin Pharmacol 2004; 44(5): 532–7
Kovarik JM, Hartmann S, Bartlett M, et al. Oral-intravenous crossover study of fingolimod pharmacokinetics, lymphocyte responses and cardiac effects. Biopharm Drug Dispos 2007; 28(2): 97–104
Kovarik JM, Schmouder RL, Barilla D, et al. FTY720 and cyclosporine: evaluation for a pharmacokinetic interaction. Ann Pharmacother 2004; 38(7–8): 1153–8
Schmouder R, Boulton C, Wang N, et al. An exploratory, randomized, double-blind, placebo controlled, parallel group, multiple dose study to assess the pharmacodynamic effect of fingolimod on antibody response following multiple immunizations in healthy volunteers. Cambridge: LCG Bioscience, 2010. (Data on file)
Ettenger R, Schmouder R, Kovarik JM, et al. Pharmacokinetics, pharmacodynamics, safety and tolerability of single-dose fingolimod (FTY720) in adolescents with stable renal transplants. Pediatr Transplant 2011; 15(4): 406–13
Kovarik JM, Tedesco-Silva H, Lorber MI, et al. Exposure-efficacy relationships of a fingolimod-everolimus regimen in kidney transplant patients at risk for delayed graft function. Transplant Proc 2006; 38(10): 3479–82
Kovarik JM, Dole K, Riviere GJ, et al. Ketoconazole increases fingolimod blood levels in a drug interaction via CYP4F2 inhibition. J Clin Pharmacol 2009; 49(2): 212–8
Collins W, Francis G, Koren G, et al. Lack of interaction between fingolimod (FTY720) and oral contraceptives, and pregnancy experience in the clinical program of fingolimod in multiple sclerosis [abstract]. Neurology 2011; 76: P07.184
Kovarik JM, Lu M, Riviere GJ, et al. The effect on heart rate of combining single-dose fingolimod with steady-state atenolol or diltiazem in healthy subjects. Eur J Clin Pharmacol 2008; 64(5): 457–63
Kappos L, Antel J, Comi G, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med 2006; 355(11): 1124–40
Kovarik JM, Riviere GJ, Neddermann D, et al. A mechanistic study to assess whether isoproterenol can reverse the negative chronotropic effect of fingolimod. J Clin Pharmacol 2008; 48(3): 303–10
Kovarik JM, Slade A, Riviere GJ, et al. The ability of atropine to prevent and reverse the negative chronotropic effect of fingolimod in healthy subjects. Br J Clin Pharmacol 2008; 66(2): 199–206
Acknowledgements
All authors are employees of Novartis and have no other conflicts of interest. The research described here was funded by Novartis, and the sponsor was involved in the design and conduct of the studies, collection of data, and analysis and interpretation of the data. The authors thank Markus Zollinger, Hans-Peter Gschwind and Yi Jin (Drug Metabolism and Pharmacokinetics, Novartis) for managing the preclinical development of fingolimod, and François Mercier, Heinz Schmidli and Marina Savelieva-Praz (Modelling and Simulation, Novartis) for conducting the population pharmacokinetics analyses of fingolimod. The authors take full responsibility for the content of the paper and thank Rowena Hughes and Sarah Griffiths of Oxford PharmaGenesis™ Ltd for editorial assistance, collating and incorporating comments from all authors, and editing the final manuscript. The editorial support was funded by Novartis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
David, O.J., Kovarik, J.M. & Schmouder, R.L. Clinical Pharmacokinetics of Fingolimod. Clin Pharmacokinet 51, 15–28 (2012). https://doi.org/10.2165/11596550-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11596550-000000000-00000