Summary
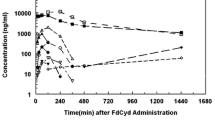
The plasma and cellular pharmacology of 2′, 2′-difluorodeoxycytidine (dFdC, Gemcitabine) was studied during a phase I trial. The steady-state concentration of dFdC in plasma was directly proportional to the dFdC dose, which ranged between 53 and 1,000 mg/m2 per 30 min. The cellular pharmacokinetics of an active metabolite, dFdC 5′-triphosphate (dFdCTP), were determined in mononuclear cells of 22 patients by anion-exchange highpressure liquid chromatography. The rate of dFdCTP accumulation and the peak cellular concentration were highest at a dose rate of 350 mg/m2 per 30 min, during which steady-state dFdC levels of 15–20 μM were achieved in plasma. A comparison of patients infused with 800 mg/m2 over 60 min with those receiving the same dose over 30 min demonstrated that the dFdC steady-state concentrations were proportional to the dose rate, but that cellular dFdCTP accumulation rates were similar at each dose rate. At the lower dose rate, the AUC for dFdCTP accumulation was 4-fold that observed at the higher dose rate. Consistent with these observations, the accumulation of dFdCTP by mononuclear cells incubated in vitro was maximal at 10–15 μM dFdC. These studies suggest that the ability of mononuclear cells to use dFdC for triphosphate formation is saturable. In the design of future protocols, a dose rate should be considered that produces maximal nucleotide analogue formation, with increased intensity being achieved by prolonging the duration of infusion.
Similar content being viewed by others
Abbreviations
- ara-C :
-
I-β-d-arabinosylcytosine
- ara-C ss :
-
steady-state concentration of ara-C
- ara-CTP :
-
5′-triphosphate of ara-C
- dFdC :
-
2′, 2′-difluorodeoxycytidine, Gemcitabine
- dFdC ss :
-
steady-state concentration of dFdC
- dFdCTP :
-
5′-triphosphate of dFdC
References
Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Saterlee W, Raber MN, Plunkett W (1990) A phase I clinical, plasma and cellular pharmacology study of 2′, 2′-difluorodeoxycytidine. J Clin Oncol (in press)
Chou T-C, Arlin Z, Clarkson BD, Philips FS (1977) Metabolism of 1-β-d-arabinofuranosylcytosine in human leukemic cells. Cancer Res 37: 3561–3570
Estey E, Keating MJ, McCredie KB, Freireich EJ, Plunkett W (1990) Cellular ara-CTP pharmacokinetics, response, and karyotype in newly diagnosed acute myelogenous leukemia. Leukemia 4: 95–99
Gandhi V, Plunkett W (1990) Modulatory activity of 2′, 2′-difluorodeoxycytidine on the phosphorylation and cytotoxicity of arabinosyl nucleosides. Cancer Res 50: 3675–3680
Grunewald R, Kantarjian H, Keating MJ, Abbruzzese J, Tarasoff P, Plunkett W (1990) Pharmacologically directed design of the dose rate and schedule of 2′, 2′-difluorodeoxycytidine administration in leukemia. Cancer Res 50: 6822–6826
Harris AL, Grahame-Smith DG (1979) Variation in sensitivity of DNA synthesis to ara-C in acute myeloid leukemia. Br J Haematol 45: 371–379
Heinemann V, Hertel LW, Grindey GB, Plunkett W (1988) Comparison of the cellular pharmacokinetics and toxicity of 2′, 2′-difluorodeoxycytidine and 1-β-d-arabinofuranosylcytosine. Cancer Res 48: 4024–4031
Heinemann V, Estey E, Keating MJ, Plunkett W (1989) Patient-specific dose rate of continuous infusion high-dose cytarabine in relapsed acute myelogenous leukemia. J Clin Oncol 7: 622–628
Heinemann V, Xu YZ, Chubb S, Sen A, Hertel L, Grindey GB, Plunkett W (1990) Inhibition of ribonucleotide reduction in CCRFCEM cells by 2′, 2′-difluorodeoxycytidine. Mol Pharmacol 38: 556–572
Hertel LW, Kroin JS, Misner JW, Tustin JM (1988) Synthesis of 2-deoxy-2,2-difluoro-d-ribose and 2-deoxy-2,2-ribofuranosyl nucleosides. J Org Chem 53: 2406–2409
Hertel LW, Boder GB, Kroin JS, Rinzel SM, Poore GA, Todd GC, Grindey GB (1990) Evaluation of the antitumor activity of gemcitabine (2′, 2′-difluoro-2′-deoxycytidine). Cancer Res 50: 4417–4422
Huang P, Chubb S, Hertel LW, Plunkett W (1990) Mechanism of action of 2′, 2′-difluorodeoxycytidine triphosphate on DNA synthesis. Proc Am Assoc Cancer Res 31: 426
Kantarjian HM, Estey EH, Plunkett W, Keating MJ, Walters RS, Iacoboni S, McCredie KB, Freireich EJ (1986) Phase-I-II clinical and pharmacologic studies of high-dose cytosine arabinoside in refractory leukemia. Am J Med 81: 387–394
Muus P, Dreth-Schonk A, Haanen C, Wessels H, Linssen P (1987) In-vitro studies on phosphorylation and dephosphorylation of cytosine arabinoside in human leukemia cells. Leukemia Res 11: 319–325
Plunkett W, Iacoboni S, Estey E, Danhauser L, Liliemark JO, Keating MJ (1985) Pharmacologically directed ara-C therapy for refractory leukemia. Semin Oncol 12 [Suppl 3]: 20–30
Plunkett W, Liliemark JO, Adams TM, Nowak B, Estey E, Kantarjian H, Keating MJ (1987) Saturation of 1-β-d-arabinofuranosyl-cytosine 5′-triphosphate accumulation in leukemia cells during highdose 1-β-d-arabinofuranosylcytosine therapy. Cancer Res 47: 3005–3011
Plunkett W, Liliemark JO, Estey E, Keating MJ (1987) Saturation of ara-CTP accumulation during high-dose ara-C therapy: pharmacologic rationale for intermediate-dose ara-C. Semin Oncol 14: [Suppl 1]: 159–166
Plunkett W, Gandhi V, Chubb S, Nowak B, Heinemann V, Mineishi S, Sen A, Hertel LW, Grindey GB (1989) 2′, 2′-Difluorodeoxycytidine metabolism and mechanism of action in human leukemia cells. Nucleosides Nucleotides 8: 775–782
Sunkara PS, Snyder RS, Jarvi ET, Farr RA (1988) Antitumor activity of 2′-deoxy-2′, 2′-difluorocytidine, a novel inhibitor of ribonucleotide reductase. Proc Am Assoc Cancer Res 29: 324
Author information
Authors and Affiliations
Additional information
Supported in part by grants CA28596 and CA32839 from the National Cancer Institute, Department of Health and Human Services, and by grant CH-130 from the American Cancer Society
Rights and permissions
About this article
Cite this article
Grunewald, R., Abbruzzese, J.L., Tarassoff, P. et al. Saturation of 2′, 2′-difluorodeoxycytidine 5′-triphosphate accumulation by mononuclear cells during a phase I trial of gemcitabine. Cancer Chemother. Pharmacol. 27, 258–262 (1991). https://doi.org/10.1007/BF00685109
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00685109