Abstract
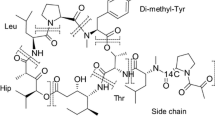
We have developed and validated a selective analytical procedure, based on ion-exchange normal-phase liquid chromatography with fluorescence detection and liquid-liquid extraction, for the analysis of vinblastine (VBL) in biological matrices. The assay is suitable for the determination of the parent compound and its metabolites in plasma, tissue, faeces and urine specimens. Pharmacokinetics studies were performed in male FVB mice receiving VBL by intravenous (i.v.) bolus injection at a dose of 6 mg/kg. Plasma concentrations were monitored until 48 h after drug administration. Urine and faeces samples were collected in 24-h portions for up to 72 h and tissue samples were obtained at 4, 24, 72 and 168 h after drug administration. To facilitate a comparison between the findings we obtained by high-performance liquid chromatography (HPLC) and the results of previous studies using radiolabeled drug monitoring, some of the animals were also given radiolabeled drug. Large discrepancies were observed between the results obtained by the two methods. Excretion of the radiolabel in faeces and urine was 85% of the dose within 72 h. HPLC revealed that only 18% of the dose was excreted as unchanged drug and 19%, as measurable metabolites [O4-deacetylvinblastine (DVBL) and two unknown compounds]. In most of the tissues taken at 4 h after drug administration, virtually all of the radioactivity represented VBL or DVBL. In all tissues taken at 72 h after drug administration, however, only very little of the radioactivity remained in the form of these compounds. Following the administration, VBL and DVBL were distributed extensively to most tissues. Many tissues appeared to possess effective means of extruding the cytotoxic drug with decreasing plasma levels. However, in some organs, including those from the genital tract and lymphatic tissues, VBL and DVBL were retained for prolonged periods. Our studies confirm previous indications that selective retention may be the basis of the activity of VBL against malignant transformations derived from these tissues.
Similar content being viewed by others
References
Castle MC, Mead JAR (1978) Investigations of the metabolic fate of tritiated vincristine in the rat by high-pressure liquid chromatography. Biochem Pharmacol 27:37–44
Castle MC, Margileth DA, Oliverio VT (1976) Distribution and excretion of [3H]vincristine in the rat and the dog. Cancer Res 36: 3684–3689
Culp HW, Daniels WD, McMahon RE (1977) Disposition and tissue levels of [3H]vindesine in rats. Cancer Res 37:3053–3056
El Dareer SM, White VM, Chen FP, Mellett LB, Hill DL (1977) Distribution and metabolism of vincristine in mice, rats, dogs and monkeys. Cancer Treat Rep 61:1269–1277
Houghton JA, Williams LG, Torrance PM, Houghton PJ (1984) Determinants of intrinsic sensitivity to vinca alkaloids in xenografts of pediatric rhabdomyosarcomas. Cancer Res 44:582–590
Noble RL, Gout PW, Wijcik LL, Hebden HF, Beer CT (1977) The distribution of [3H]vinblastine in tumor and host tissues of Nb rats bearing a transplantable lymphoma which is highly sensitive to the alkaloid. Cancer Res 37:1455–1460
Owellen RJ, Hartke CA, Haines FO (1977) Pharmacokinetics and metabolism of vinblastine in humans. Cancer Res 37:2597–2602
Rahmani R, Zhou XJ, Placidi M, Martin M, Cano JP (1990) In vivo and in vitro pharmacokinetics and metabolism of vinca alkaloids in rat: I. Vindesine (4-deacetyl-vinblastine-3-carboxyamide). Eur J Drug Metab Pharmacokinet 15:49–55
Van Tellingen O, Beijnen JH, Baurain R, Ten Bokkel Huinink WW, Van der Woude HR, Nooijen WJ (1991) High performance liquid chromatography analysis of vinblastine, 4-O-deacetylvinblastine and the potential metabolite 4-O-deacetylvinblastine-3-oic acid in biological fluids. J Chromatogr 553:47–53
Van Tellingen O, Beijnen JH, Nooijen WJ (1991) Analytical methods for the determination of vinca alkaloids in biological specimens: a survey of the literature. J Pharm Biomed Anal 9:1077–1082
Van Tellingen O, Sips JHM, Beijnen JH, Bult A, Nooijen WJ (1992) Pharmacology, bioanalysis and pharmacokinetics of the vinca alkaloids and semisynthetic derivatives (review). Anticancer Res 12: 1699–1715
Vendrig DEMM (1989) Bio-analysis, stability, pharmacokinetics and electrochemistry of vinca alkaloids. Thesis, University of Utrecht
Zhou XJ, Martin M, Placidi M, Cano JP, Rahmani R (1990) In vivo and in vitro pharmacokinetics and metabolism of vinca alkaloids in rat: II. Vinblastine and vincristine. Eur J Drug Metab Pharmacokinet 15:323–332
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Tellingen, O., Beijnen, J.H., Nooijen, W.J. et al. Tissue disposition, excretion and metabolism of vinblastine in mice as determined by high-performance liquid chromatography. Cancer Chemother. Pharmacol. 32, 286–292 (1993). https://doi.org/10.1007/BF00686174
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00686174