Summary
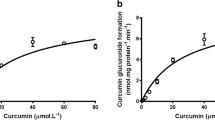
The aim of this study was to investigate the influence of several anticancer drugs and investigational multidrug resistance (MDR) reversing agents on the hepatic metabolism of paclitaxel (Taxol) to its primary metabolites, 6 α-hydroxypaclitaxel (metabolite, MA) and 3′-p-hydroxypaclitaxel (metabolite, MB). There is significant inter-individual variability associated with the levels of these two metabolites. In many cases, 6α-hydroxypaclitaxel has been observed to be the predominant metabolite, in others, 3′-p-hydroxypaclitaxel has been the principal metabolite. The formation of 6α-hydroxypaclitaxel and 3′-p-hydroxypaclitaxel is catalyzed by cytochrome P450 isozymes CYP2C8 and CYP3A4, respectively. A number of factors, including co-administration of drugs and adjuvants, are known to influence the activity of these isozymes. Therefore, the influence of MDR reversing agents, R-verapamil, cyclosporin A (CsA) and tamoxifen and anti-cancer drugs doxorubicin, etoposide (VP-16) and cisplatin on paclitaxel metabolism was assessed employing human liver microsomes in vitro. Paclitaxel (10 μM) was incubated with human liver microsomes (1 mg protein, −0.34 nmol CYP) in the presence of a NADPH generating system at 37°C for 1 h, with and without the presence of interacting drug. Controls included incubations with quercetin and ketoconazole, known inhibitors of 6α-hydroxypaclitaxel and 3′-p-hydroxypaclitaxel formation, respectively. At the end of the incubation period, paclitaxel and the metabolites were extracted in ethyl acetate and analyzed employing an HPLC method. Significant inhibition of paclitaxel conversion to 6α-hydroxypaclitaxel and 3′-p-hydroxypaclitaxel was observed in the presence of R-verapamil, tamoxifen and VP-16 (P 0.005). Doxorubicin significantly inhibited the formation of 3′-p-hydroxypaclitaxel and CsA inhibited the formation of 6α-hydroxypaclitaxel (P 0.005). This study demonstrates that co-administration of several of the above listed compounds could lead to significant changes in the pharmacokinetics of paclitaxel.
Similar content being viewed by others
References
Schiff P.B., Fant J., Horwitz S. (1979): Promotion of microtubule assembly in vitro by taxol. Nature, 277, 665–667.
Rowinsky E.K., Gilbert M.R., McGuire W.P. et al. (1991): Sequences of taxol and cisplatin: a phase I and pharmacologic studies. J. Clin. Oncol., 9, 1692–1703.
Sledge G.W., Robert N., Sparano J.A. et al. (1994): Paclitaxel (Taxol)/doxorubicin combinations in advanced breast cancer: The Eastern Cooperative Oncology Group experience. Semin. Oncol., 21 (suppl. 8), 15–18.
Hortobagyi G.N. (1997): Paclitaxel-based combination chemotherapy for breast cancer. Oncology, XX, 29–37.
Cresteil T., Monsarrat B., Alvinerie P., Tréluyer J.M.T., Vierira I., Wright M. (1994): Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res., 54, 386–392.
Kumar G.N., Walle U.K., Walle T. (1994): Cytochrome P450 3A-mediated human liver microsomal taxol 6 alpha-hydroxylation. J. Pharmacol. Exp. Ther., 268, 1160–1165.
Harris J.W., Rahman A., Kim B-R., Guengerich F.P., Collins J.M. (1994): Metabolism of Taxol by human hepatic microsomes and liver slices: participation of cytochrome P450 3A4 and an unknown P450 enzyme. Cancer Res., 54, 4026–4035.
Rahman A., Korekwa K.R., Grogan J., Gonzalez F.J., Harris J.W. (1994): Selective biotransformation of taxol to 6α-hydroxytaxol by human cytochrome P450 2C8. Cancer Res., 54, 5543–5546.
Walle T., Walle U.W., Kumar G.N., Bhalla K.N. (1995): Taxol metabolism and disposition in cancer patients. Drug Metab. Dispos., 23, 506–512.
Sonnichsen D.S., Liu Q., Schuetz E.G., Schuetz J.D., Pappo A., Relling M.V. (1995): Variability in human cytochrome P450 paclitaxel metabolism. J. Pharmacol. Exp. Ther., 275, 566–575.
Sonnichsen D.S., Hurwitz C.A., Pratt C.B., Shuster J.J., Relling M.V. (1994): Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J. Clin. Oncol. 12, 532–538.
Beijnen J.H., Huizing M.T., ten Bokkel W.W. et al. (1994): Bioanalysis, pharmacokinetics and pharmacodynamics of the novel anti-cancer drug paclitaxel (Taxol). Semin. Oncol., 21 (suppl 8), 53–62.
Berg S.L., Tolcher A., O’Shaughnessy J.A. et al. (1995): Effect of R-verapamil on the pharmacokinetics of paclitaxel in women with breast cancer. J. Clin. Oncol., 13, 2039–2042.
Huizing M.T., Sparreboom A., Rosing H., van Tellingen O., Pinedo H.M., Beijnen J.H. (1995): Quantification of paclitaxel metabolites in human plasma by high-performance liquid chromatography. J. Chromatogr. B: Biomed Appl., 674, 261–268.
Kumar G., Ray S., Walle T. et al. (1995): Comparative in vitro cytotoxic effects of taxol and its major human metabolite 6α-hydroxypaclitaxel. Cancer Chemother. Pharmacol., 36, 129–135.
Juranka P.F., Zastawny R.I., Ling V. (1989): P-glycoprotein: multidrug resistance and a superfamily of membrane-associated transport proteins. FASEB J., 3: 2583–2592.
Horwitz S.B., Lothstein L., Manfredi J.J. et al. (1986): Taxol: Mechanisms of action and resistance. Ann. N.Y. Acad. Sci., 466, 733–744.
Lum B.L., Fisher G.A., Brophy N.A. et al. (1993): Clinical trials of modulators of multidrug resistance: pharmacokinetic and pharmacodynamic consideration. Cancer, 72, 3502–3514.
Stuart N.S.A., Philip, P., Harris A.L. et al. (1992): High-dose tamoxifen as an enhancer of etoposide cytotoxicity. Clinical effects and in vitro assessment in P-glycoprotein expressing cell lines. Br. J. Cancer, 66, 833–839.
Buckley M.M-T., Goa K.L., Tamoxifen. (1989): A re-appraisal of its pharmacodynamics and pharmacokinetic properties, and therapeutic use. Drugs, 37, 451–490.
Royer I., Monsarrat B., Sonnier M., Wright M., Cresteil T. (1996): Metabolism of docetaxel by human cytochrome P450: interactions with paclitaxel and other anti-neoplastic agents. Cancer Res., 56, 58–65.
Lum B.L., Kaubisch S., Yahanda A.M. et al. (1992): Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporin in a phase I trial to modulate multidrug resistance. J. Clin. Oncol., 10, 1635–1642.
Mani C., Gelboin H.V., Park S.S., Pearce R., Parkinson A., Kupfe D. (1993): Metabolism of the antimammary cancer anti-estrogenic agent tamoxifen. I. Cytochrome P-450-catalyzed N-demethylation and 4-hydroxylation. Drug Metab. Dispos., 21, 645–656.
Kroemer H.K., Gautier J.C., Beaune P., Henderson C., Wolf C.R., Eichelbaum M. (1993): Identification of P450 enzymes involved in metabolism of verapamil in humans. Naunyn Schmiedebergs Arch. Pharmacol., 348, 332–337.
Lemoine A., Azoulay D., Gries J.M. et al. (1993): Relationship between graft cytochrome P-450 3A content and early morbidity after liver transplantation. Transplantation, 56, 1410–1414.
Leblanc G.A., Sundseth S.S., Weber G.F., Waxman D.J. (1992): Platinum anti-cancer drugs modulate P450 mRNA levels and differentially alter hepatic drug and steroid metabolism in male and female rats. Cancer Res., 52, 540–547.
Relling M.V., Nemec J., Schuetz, E.G., Schuetz J.D., Gonzalez F.J., Korzekwa K.R. (1994): O-demethylation of epidophyllotoxins is catalyzed by human cytochrome P450 3A4. Mol. Pharmacol., 45, 352–358.
Kivisko K.T., Kroemer H.K., Eichelbaum M. (1995): The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implication for drug interactions. Br. J. Clin. Pharmacol., 40, 523–530.
Lien E.A., Solheim E., Ueland P.M. (1991): Distribution of tamoxifen and its metabolites in rat and human tissues during steady-state treatment. Cancer Res., 51, 4837–4844.
Hamann S.R., Todd G.R., McAllister R.G. (1983): The pharmacology of verapamil. V. Tissue distribution of verapamil and norverapamil in rat and dog. Pharmacology, 27, 1–8.
Lesser G.J., Grossman S.A., Eller S., Rowinsky E.K. (1995): The distribution of systemically administered [3H]-paclitaxel in rats: a quantitative autoradiographic study. Cancer Chemother. Pharmacol., 37, 173–178.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Desai, P.B., Duan, J.Z., Zhu, Y.W. et al. Human liver microsomal metabolism of paclitaxel and drug interactions. Eur. J. Drug Metab. Pharmacokinet. 23, 417–424 (1998). https://doi.org/10.1007/BF03192303
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF03192303