Abstract
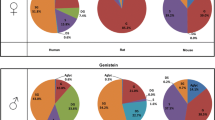
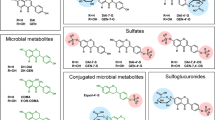
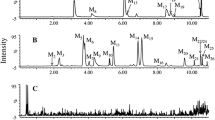
Much attention has been paid to the metabolism and disposition of isoflavones daidzein (Dein) and genistein (Gein) with regard to the prevention of several hormone-dependent diseases. Recent studies have reported that several conjugates as well as aglycones may be biologically active or may be activated within target cells. However, the disposition of Dein and Gein in plasma is still uncertain. This paper describes the identification and quantification of the highly polar metabolites, daidzein-7-glucuronide-4′-sulfate (D-7G-4′S), genistein-7-glucuronide-4′-sulfate (G-7G-4′S), daidzein-4′,7-diglucuronide (D-4′,7-diG), and genistein-4′,7-diglucuronide (G-4′,7-diG) in human plasma after dietary administration of kinako (baked soybean powder) to two healthy volunteers. The structure identification of these conjugated metabolites in plasma was performed in comparison to the LC-ESI-MS and 600 MHz 1H-NMR spectral data of the chemically synthesized compounds. Furthermore, 16 isoflavone metabolites including D-7G-4′S, G-7G-4′S, D-4′,7-diG, and G-4′,7-diG in plasma were simultaneously measured by a high-performance liquid chromatography–UV-diode-array detector method combined with solid-phase extraction using an Oasis HLB cartridge. D-7G-4′S, G-7G-4′S and G-4′,7-diG were found to be major metabolites of Dein and Gein in plasma, while intact aglycones were detected to be only ca. 2% in both subjects. The findings suggest that the conjugated metabolites could be the key compounds responsible for pharmacological and medicinal properties of isoflavones.






Similar content being viewed by others
References
Adlercreutz H (2002) Lancet Oncol 3:364–373
Setchell KDR, Lydeking-Olsen E (2003) Am J Clin Nutr 78:593S–609S
McCue P, Shetty K (2004) Crit Rev Food Sci Nutr 44:361–367
Chang ET, Lee VS, Canchola AJ, Clarke CA, Purdie DM, Reynolds P, Anton-Culver H, Bernstein L, Deapen D, Peel D, Pinder R, Ross RK, Stram DO, West DW, Wright W, Ziogas A, Horn-Ross PL (2007) Am J Epidemiol 165:802–813
Clarke DB, Bailey V, Lloyd AS (2008) Food Addit Contam Part A Chem Anal Control Expo Risk Assess 25:534–547
Németh K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA (2003) Eur J Nutr 42:29–42
Heinonen SM, Wähälä K, Liukkonen KH, Aura AM, Poutanen K, Adlercreutz H (2004) J Agric Food Chem 52:2640–2646
Chang YC, Nair MG (1995) J Nat Prod 58:1892–1896
Adlercreutz H, van der Wildt J, Kinzel J, Attalla H, Wähälä K, Mäkelä T, Hase T, Fotsis T (1995) J Steroid Biochem Mol Biol 52:97–103
Zhang Y, Hendrich S, Murphy PA (2003) J Nutr 133:399–404
Wong CK, Keung WM (1997) Biochem Biophys Res Commun 233:579–583
Zhang Y, Song TT, Cunnick JE, Murphy PA, Hendrich S (1999) J Nutr 129:399–405
Morand C, Manach C, Donovan J, Remesy C (2001) Methods Enzymol 335:115–121
Adlercreutz H, Fotsis T, Watanabe S, Lampe J, Wähälä K, Mäkelä T, Hase T (1994) Cancer Detect Prev 18:259–271
Maskarinec G, Yamakawa R, Hebshi S, Franke AA (2007) Eur J Clin Nutr 61:255–261
Hosoda K, Furuta T, Yokokawa A, Ogura K, Hiratsuka A, Ishii K (2008) Drug Metab Dispos 36:1485–1495
Needs PW, Williamson G (2001) Carbohydr Res 330:511–515
Nakano H, Ogura K, Takahashi E, Harada T, Nishiyama T, Muro K, Hiratsuka A, Kadota S, Watabe T (2004) Drug Metab Pharmacokinet 19:216–226
Soidinsalo O, Wähälä K (2007) Steroids 72:851–854
Jacquinet JC (1990) Carbohydr Res 199:153–181
Hosoda K, Furuta T, Ishii K (2010) J Chromatogr B Analyt Technol Biomed Life Sci 878:628–636
Yasuda T, Mizunuma S, Kano Y, Saito K, Ohsawa K (1996) Biol Pharm Bull 19:413–417
Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM (2002) Am J Clin Nutr 76:588–594
Cassidy A (2006) J AOAC Int 89:1182–1188
Gu L, House SE, Prior RL, Fang N, Ronis MJ, Clarkson TB, Wilson ME, Badger TM (2006) J Nutr 136:1215–1221
Clarke DB, Lloyd AS, Botting NP, Oldfield MF, Needs PW, Wiseman H (2002) Anal Biochem 309:158–172
Tsuchihashi R, Okawa M, Nohara T, Okabe H, Kinjo J (2004) Nat Med 58:71–75
Acknowledgments
We thank Masashi Miyawaki and Mei Ying Han (Thermo Fisher Scientific, Kanagawa, Japan) for support of the LC-ESI-MS analyses. This study was supported by a Project Research Grant from Kyorin University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosoda, K., Furuta, T., Yokokawa, A. et al. Identification and quantification of daidzein-7-glucuronide-4′-sulfate, genistein-7-glucuronide-4′-sulfate and genistein-4′,7-diglucuronide as major metabolites in human plasma after administration of kinako . Anal Bioanal Chem 397, 1563–1572 (2010). https://doi.org/10.1007/s00216-010-3714-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-010-3714-8