Abstract
Objective
The aim of this study was to evaluate the absolute bioavailability and the metabolism of omeprazole following single intravenous and oral administrations to healthy subjects in relation to CYP2C19 genotypes.
Methods
Twenty subjects, of whom 6 were homozygous extensive metabolizers (hmEMs), 8 were heterozygous EMs (htEMs) and 6 were poor metabolizers (PMs) for CYP2C19, were enrolled in this study. Each subject received either a single omeprazole 20 mg intravenous dose (IV) or 40 mg oral dose (PO) in a randomized fashion during 2 different phases.
Results
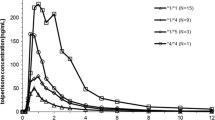
Mean omeprazole AUC (0,∞) was 1164, 3093 and 10511 ng h/mL after PO, and 1435, 2495 and 6222 ng h/mL after IV in hmEMs, htEMs and PMs, respectively. Therefore, the absolute bioavailability of omeprazole in PMs was significantly higher than that in hmEMs (p < 0.001) and htEMs (p < 0.001). Hydroxylation metabolic indexes after IV and PO were significantly lower in PMs than in hmEMs (p < 0.001) and htEMs (p < 0.001), and was correlated with the absolute bioavailability (p < 0.0001 for both IV and PO). Sulfoxidation metabolic index after IV was significantly different between the CYP2C19 genotypes, whereas no difference was found after a single oral dose.
Conclusion
This study indicates that the absolute bioavailability of omeprazole differs among the three different CYP2C19 genotypes after a single dose of omeprazole orally or intravenously. Hydroxylation metabolic index of omeprazole may be mainly attributable to the genotype of CYP2C19. As for the sulfoxidation metabolic index after a single oral dose, intestinal CYP3A may be contributed to omeprazole metabolism.

Similar content being viewed by others
References
Wallmark B, Jaresten BM, Larsson H, Ryberg B, Brandstrom A, Fellenius E (1983) Differentiation among inhibitory actions of omeprazole, cimetidine, and SCN- on gastric acid secretion. Am J Physiol 245:G64–G71
Howden CW (1991) Clinical pharmacology of omeprazole. Clin Pharmacokinet 20:38–49
Maton PN (1991) Omeprazole. N Engl J Med 324:965–975
Lind T, Megraud F, Unge P, Bayerdorffer E, O’morain C, Spiller R, Veldhuyzen Van Zanten S, Bardhan KD, Hellblom M, Wrangstadh M, Zeijlon L, Cederberg C (1999) The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology 116:248–253
Tytgat GN (1994) Review article: treatments that impact favourably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther 8:359–368
Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdorffer E, O’Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M, Cederberg C, Idstrom JP (1996) Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter 1:138–144
Chiba K, Kobayashi K, Manabe K, Tani M, Kamataki T, Ishizaki T (1993) Oxidative metabolism of omeprazole in human liver microsomes: cosegregation with S-mephenytoin 4′-hydroxylation. J Pharmacol Exp Ther 266:52–59
Andersson T, Miners JO, Veronese ME, Birkett DJ (1994) Identification of human liver cytochrome P450 isoforms mediating secondary omeprazole metabolism. Br J Clin Pharmacol 37:597–604
Kita T, Tanigawara Y, Aoyama N, Hohda T, Saijoh Y, Komada F, Sakaeda T, Okumura K, Sakai T, Kasuga M (2001) CYP2C19 genotype related effect of omeprazole on intragastric pH and antimicrobial stability. Pharm Res 18:615–621
Andersson T, Regardh CG, Lou YC, Zhang Y, Dahl ML, Bertilsson L (1992) Polymorphic hydroxylation of S-mephenytoin and omeprazole metabolism in Caucasian and Chinese subjects. Pharmacogenetics 2:25–31
Sohn DR, Kobayashi K, Chiba K, Lee KH, Shin SG, Ishizaki T (1992) Disposition kinetics and metabolism of omeprazole in extensive and poor metabolizers of S-mephenytoin 4′-hydroxylation recruited from an Oriental population. J Pharmacol Exp Ther 262:1195–1202
Chang M, Tybring G, Dahl ML, Gotharson E, Sagar M, Seensalu R, Bertilsson L (1995) Disposition kinetics and metabolism of omeprazole in extensive and poor metabolizers of S-mephenytoin 4′-hydroxylation recruited from an Oriental population. Br J Clin Pharmacol 39:511–518
Ieiri I, Kubota T, Urae A, Kimura M, Wada Y, Mamiya K, Yoshioka S, Irie S, Amamoto T, Nakamura K, Nakano S, Higuchi S (1996) Pharmacokinetics of omeprazole (a substrate of CYP2C19) and comparison with two mutant alleles, C gamma P2C19m1 in exon 5 and C gamma P2C19m2 in exon 4, in Japanese subjects. Clin Pharmacol Ther 59:647–653
Andersson T, Cederberg C, Regardh CG, Skanberg I (1990) Pharmacokinetics of various single intravenous and oral doses of omeprazole. Eur J Clin Pharmacol 39:195–197
Regardh CG, Andersson T, Lagerstrom PO, Lundborg P, Skanberg I (1990) The pharmacokinetics of omeprazole in humans-a study of single intravenous and oral doses. Ther Drug Monit 12:163–172
De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA (1994) Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol 46:594–859
Shimizu M, Uno T, Niioka T, Yasui-Furukori N, Takahata T, Sugawara S, Tateishi T (2006) Sensitive determination of omeprazole and its two main metabolites in human plasma by column-switching high-performance liquid chromatography: application to pharmacokinetic study in relation to CYP2C19 genotypes. J Chromatogr B 832:241–248
Andersson T (1996) Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet 31:9–28
Schwab M, Klotz U, Hofmann U, Schaeffeler E, Leodolter A, Malfertheiner P, Treiber G (2005) Esomeprazole-induced healing of gastroesophageal reflux disease is unrelated to the genotype of CYP2C19: evidence from clinical and pharmacokinetic data. Clin Pharmacol Ther 78:627–634
Lapple F, von Richter O, Fromm MF, Richter T, Thon KP, Wisser H, Griese EU, Eichelbaum M, Kivisto KT (2003) Differential expression and function of CYP2C isoforms in human intestine and liver. Pharmacogenetics 13:565–575
Andersson T, Miners JO, Veronese ME, Birkett DJ (1994) Identification of human liver cytochrome P450 isoforms mediating secondary omeprazole metabolism. Br J Clin Pharmacol 37:597–604
Watkins PB, Wrighton SA, Schuetz EG, Molowa DT, Guzelian PS (1987) Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J Clin Invest 80:1029–1036
Pauli-Magnus C, Rekersbrink S, Klotz U, Fromm MF (2001) Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn Schmiedebergs Arch Pharmacol 364:551–557
Andersson T, Holmberg J, Rohss K, Walan A (1998) Pharmacokinetics and effect on caffeine metabolism of the proton pump inhibitors, omeprazole, lansoprazole, and pantoprazole. Br J Clin Pharmacol 45:369–375
Yasuda S, Horai Y, Tomono Y, Nakai H, Yamato C, Manabe K, Kobayashi K, Chiba K, Ishizaki T (1995) Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4′-hydroxylation status. Clin Pharmacol Ther 58:143–154
Furuta T, Shirai N, Sugimoto M, Ohashi K, Ishizaki T (2004) Pharmacogenomics of proton pump inhibitors. Pharmacogenomics 5:181–202
Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L (2000) Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos 28:966–972
Kanazawa H, Okada A, Higaki M, Yokota H, Mashige F, Nakahara K (2003) Stereospecific analysis of omeprazole in human plasma as a probe for CYP2C19 phenotype. J Pharm Biomed Anal 30:1817–1824
Acknowledgment
We thank Dr. Tommy Andersson (Experimental Medicine, AstraZeneca LP, Wilmington, USA) for meaningful advice in the discussion section. This work was supported by the Japanese Research Foundation for Clinical Pharmacology.
Conflict of interest statement
The authors have no conflicts of interest in relation to this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uno, T., Niioka, T., Hayakari, M. et al. Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol 63, 143–149 (2007). https://doi.org/10.1007/s00228-006-0251-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-006-0251-7