Abstract
Purpose
We investigated the population pharmacokinetics and exposure-response relationship of nilotinib in patients with newly diagnosed chronic myeloid leukemia (CML) in chronic phase.
Methods
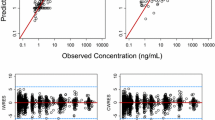
Nilotinib was given at 300 mg or 400 mg twice daily. Serum concentration data (sparse and full pharmacokinetic profiles) were obtained from 542 patients over 12 months. A population pharmacokinetic analysis was performed using nonlinear mixed-effect modeling. Exposure-response relationships were explored graphically or using logistic regression models.
Results
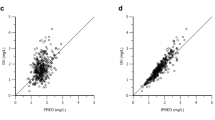
Nilotinib concentrations were stable over 12 months. Patients in the 400 mg twice-daily arm had an 11.5% higher exposure than did those in the 300 mg twice-daily arm, and the relative bioavailability of nilotinib 400 mg twice daily was 0.84 times that of 300 mg twice daily. Patient demographics did not significantly affect nilotinib pharmacokinetics. The occurrence of all-grade total bilirubin elevation was significantly higher in patients with higher nilotinib exposure, and a positive correlation was also observed between nilotinib exposure and QTcF change on electrocardiograms from baseline. There was no significant relationship between nilotinib exposure and major molecular response at 12 months.
Conclusions
There is a less than proportional dose-exposure relationship between nilotinib 300 mg and 400 mg twice-daily doses. Blood level testing is unlikely to play an important role in the general management of patients with newly diagnosed CML treated with nilotinib.





Similar content being viewed by others
References
Novartis (2010) Tasigna [package insert]. Novartis Pharmaceuticals, East Hanover, NJ
Weisberg E, Manley P, Mestan J, Cowan-Jacob S, Ray A, Griffin JD (2006) AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer 94(12):1765–1769
Manley PW, Drueckes P, Fendrich G, Furet P, Liebetanz J, Martiny-Baron G, Mestan J, Trappe J, Wartmann M, Fabbro D (2010) Extended kinase profile and properties of the protein kinase inhibitor nilotinib. Biochim Biophys Acta 1804(3):445–453
Weisberg E, Manley PW, Breitenstein W, Bruggen J, Cowan-Jacob SW, Ray A, Huntly B, Fabbro D, Fendrich G, Hall-Meyers E, Kung AL, Mestan J, Daley GQ, Callahan L, Catley L, Cavazza C, Azam M, Neuberg D, Wright RD, Gilliland DG, Griffin JD (2005) Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell 7(2):129–141
Rosti G, Palandri F, Castagnetti F, Breccia M, Levato L, Gugliotta G, Capucci A, Cedrone M, Fava C, Intermesoli T, Cambrin GR, Stagno F, Tiribelli M, Amabile M, Luatti S, Poerio A, Soverini S, Testoni N, Martinelli G, Alimena G, Pane F, Saglio G, Baccarani M, GIMEMA CML Working Party (2009) Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood 114(24):4933–4938
Cortes JE, Jones D, O'Brien S, Jabbour E, Konopleva M, Ferrajoli A, Kadia T, Borthakur G, Stigliano D, Shan J, Kantarjian H (2010) Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol 28(3):392–397
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, Lechner K, Nielsen JL, Rousselot P, Reiffers J, Saglio G, Shepherd J, Simonsson B, Gratwohl A, Goldman JM, Kantarjian H, Taylor K, Verhoef G, Bolton AE, Capdeville R, Druker BJ (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348(11):994–1004
Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, Radich JP, Saglio G, Pane F, Kamel-Reid S, Wang YL, Press RD, Lynch K, Rudzki Z, Goldman JM, Hughes T (2008) Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood 112(8):3330–3338
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ, Gabert J, Grimwade D, Hehlmann R, Kamel-Reid S, Lipton JH, Longtine J, Martinelli G, Saglio G, Soverini S, Stock W, Goldman JM (2006) Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 108(1):28–37
Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, Goldman J, Hughes T (2006) Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia 20(11):1925–1930
Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, Gallagher N, Hoenekopp A, Dong M, Haque A, Larson RA, Kantarjian HM, the ENESTnd Investigators (2010) Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med 362(24):2251–2259
Tanaka C, Yin OQ, Sethuraman V, Smith T, Wang X, Grouss K, Kantarjian H, Giles F, Ottmann OG, Galitz L, Schran H (2010) Clinical pharmacokinetics of the BCR-ABL tyrosine kinase inhibitor nilotinib. Clin Pharmacol Ther 87(2):197–203
Muller MC, Cross NC, Erben P, Schenk T, Hanfstein B, Ernst T, Hehlmann R, Branford S, Saglio G, Hochhaus A (2009) Harmonization of molecular monitoring of CML therapy in Europe. Leukemia 23(11):1957–1963
Yin OQ, Gallagher N, Li A, Zhou W, Harrell R, Schran H (2010) Effect of grapefruit juice on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol 50(2):188–194
Mandema JW, Verotta D, Sheiner LB (1992) Building population pharmacokinetic—pharmacodynamic models. I. Models for covariate effects J Pharmacokinet Biopharm 20(5):511–528
Rowland M, Tozer TN (1995) Clinical pharmacokinetics: concepts and applications. Williams and Wilkins, Pittsburgh
Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG (2006) Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 354(24):2542–2551
Peng B, Hayes M, Resta D, Racine-Poon A, Druker BJ, Talpaz M, Sawyers CL, Rosamilia M, Ford J, Lloyd P, Capdeville R (2004) Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol 22(5):935–942
Wang X, Hochhaus A, Kantarjian HM, Agrawal S, Roy A, Pfister M, Chen T, Bleickardt E, Nicaise C, Shah N (2008) Dasatinib pharmacokinetics and exposure-response (E-R): relationship to safety and efficacy in patients (pts) with chronic myeloid leukemia (CML). J Clin Oncol 26(15):175s [abstract 3590]
Schmidli H, Peng B, Riviere GJ, Capdeville R, Hensley M, Gathmann I, Bolton AE, Racine-Poon A (2005) Population pharmacokinetics of imatinib mesylate in patients with chronic-phase chronic myeloid leukaemia: results of a phase III study. Br J Clin Pharmacol 60(1):35–44
Wojnowski L (2004) Genetics of the variable expression of CYP3A in humans. Ther Drug Monit 26(2):192–199
Picard S, Titier K, Etienne G, Teilhet E, Ducint D, Bernard MA, Lassalle R, Marit G, Reiffers J, Begaud B, Moore N, Molimard M, Mahon FX (2007) Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 109(8):3496–3499
Datz FL, Christian PE, Moore J (1987) Gender-related differences in gastric emptying. J Nucl Med 28(7):1204–1207
Stephen AM, Wiggins HS, Englyst HN, Cole TJ, Wayman BJ, Cummings JH (1986) The effect of age, sex and level of intake of dietary fibre from wheat on large-bowel function in thirty healthy subjects. Br J Nutr 56(2):349–361
Nicolas JM, Espie P, Molimard M (2009) Gender and interindividual variability in pharmacokinetics. Drug Metab Rev 41(3):408–421
Fujita KI, Sugiyama M, Akiyama Y, Ando Y, Sasaki Y (2011) The small-molecule tyrosine kinase inhibitor nilotinib is a potent noncompetitive inhibitor of the SN-38 glucuronidation by human UGT1A1. Cancer Chemother Pharmacol 67(1):237–241
Singer JB, Shou Y, Giles F, Kantarjian HM, Hsu Y, Robeva AS, Rae P, Weitzman A, Meyer JM, Dugan M, Ottmann OG (2007) UGT1A1 promoter polymorphism increases risk of nilotinib-induced hyperbilirubinemia. Leukemia 21(11):2311–2315
Larson RA, Hochhaus A, Saglio G, Rosti G, Lopez JL, Stenke L, Nakamae H, Goldberg SL, Wang MC, Gallagher NJ, Hoenekopp A, Ortmann CE, Hughes TP, Kantarjian HM (2010) Cardiac safety profile of imatinib and nilotinib in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): results from ENESTnd. Blood 116(21):944–945 [abstract 2291]
White DL, Saunders VA, Dang P, Engler J, Zannettino AC, Cambareri AC, Quinn SR, Manley PW, Hughes TP (2006) OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood 108(2):697–704
Davies A, Jordanides NE, Giannoudis A, Lucas CM, Hatziieremia S, Harris RJ, Jorgensen HG, Holyoake TL, Pirmohamed M, Clark RE, Mountford JC (2009) Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia 23(11):1999–2006
Larson RA, Druker BJ, Guilhot FA, O'Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y (2008) Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood 111(8):4022–4028
Acknowledgments
We thank Yen-Lin Chia, PhD, and William Sallas, PhD, for their contribution to the analyses described herewith. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. We thank Erinn Goldman, PhD, for medical editorial assistance with this manuscript.
Conflicts of interest
Dr. Larson received research support, honoraria, and consulting fees from Novartis; Dr. Yin is an employee and stock holder of Novartis; Dr. Hochhaus has received research support, honoraria, and consulting fees from Novartis and Bristol-Myers Squibb; Dr. Saglio has received honoraria and consulting fees from Novartis and Bristol-Myers Squibb; Dr. Clark has received research support, honoraria, and consulting fees from Novartis; Dr. Nakamae has received honoraria from Novartis and Bristol-Myers Squibb; Dr. Gallagher is a Novartis employee and stock holder; Dr. Eren Demirhan is a Novartis employee; Dr. Hughes has received research support, honoraria, and consulting fees from Novartis and Bristol-Myers Squibb; Dr. Kantarjian has received research support and consulting fees from Novartis; and Dr. le Coutre has received research support and honoraria from Novartis and consulting fees from Novartis and Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Additional information
Richard A. Larson and Ophelia Q. P. Yin are co-first authors.
Rights and permissions
About this article
Cite this article
Larson, R.A., Yin, O.Q.P., Hochhaus, A. et al. Population pharmacokinetic and exposure-response analysis of nilotinib in patients with newly diagnosed Ph+ chronic myeloid leukemia in chronic phase. Eur J Clin Pharmacol 68, 723–733 (2012). https://doi.org/10.1007/s00228-011-1200-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1200-7