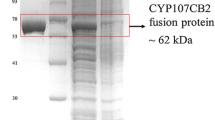
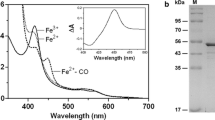
Abstract
The oxidizing activity of CYP109B1 from Bacillus subtilis was reconstituted in vitro with various artificial redox proteins including putidaredoxin reductase and putidaredoxin from Pseudomonas putida, truncated bovine adrenodoxin reductase and adrenodoxin, flavodoxin reductase and flavodoxin from Escherichia coli, and two flavodoxins from B. subtilis (YkuN and YkuP). Binding and oxidation of a broad range of chemically different substrates (fatty acids, n-alkanes, primary n-alcohols, terpenoids like (+)-valencene, α- and β-ionone, and the steroid testosterone) were investigated. CYP109B1was found to oxidize saturated fatty acids (conversion up to 99%) and their methyl and ethyl esters (conversion up to 80%) at subterminal positions with a preference for the carbon atoms C11 and C12 counted from the carboxyl group. For the hydroxylation of primary n-alcohols, the ω−2 position was preferred. n-Alkanes were not accepted as substrates by CYP109B1. Regioselective hydroxylation of terpenoids α-ionone (∼70% conversion) and β-ionone (∼ 91% conversion) yielded the allylic alcohols 3-hydroxy-α-ionone and 4-hydroxy-β-ionone, respectively. Furthermore, indole was demonstrated to inhibit fatty acid oxidation.





Similar content being viewed by others
References
Agematu H, Matsumoto N, Fujii Y, Kabumoto H, Doi S, Machida K, Ishikawa J, Arisawa A (2006) Hydroxylation of testosterone by bacterial cytochromes P450 using the Escherichia coli expression system. Biosci Biotechnol Biochem 70:307–311
Arisawa A, Agematu H (2007) A modular approach to biotransformation using microbial cytochrome P450 monooxygenases. In: Schmid RD, Urlacher VB (eds) Modern biooxidation, 1st edn. Wiley-VCH, Weinheim, pp 177–192
Bell SG, Wong LL (2007) P450 enzymes from the bacterium Novosphingobium aromaticivorans. Biochem Biophys Res Commun 360:666–672
Bell SG, Dale A, Rees NH, Wong LL (2009) A cytochrome P450 class I electron transfer system from Novosphingobium aromaticivorans. Appl Microbiol Biotechnol. doi:10.1007/s00253-009-2234-y
Bernhardt R (2006) Cytochromes P450 as versatile biocatalysts. J Biotechnol 124:128–145
Budde M, Maurer SC, Schmid RD, Urlacher VB (2004) Cloning, expression and characterisation of CYP102A2, a self-sufficient P450 monooxygenase from Bacillus subtilis. Appl Microbiol Biotechnol 66:180–186
Celik A, Flitsch SL, Turner NJ (2005) Efficient terpene hydroxylation catalysts based upon P450 enzymes derived from actinomycetes. Org Biomol Chem 3:2930–2934
Chu JW, Kimura T (1973) Studies on adrenal steroid hydroxylases. Molecular and catalytic properties of adrenodoxin reductase (a flavoprotein). J Biol Chem 248:2089–2094
Correia MA, Oritz de Montellano PR (2005) Inhibition of cytochrome P450 enzymes. In: Oritz de Montellano PR (ed) Cytochrome P450: structure, mechanism, and biochemistry, 3rd edn. Springer, Berlin, pp 247–322
Cryle MJ, Stok JE, De Voss JJ (2003) Reactions catalyzed by bacterial cytochromes P450. Aust J Chem 56:749–762
Endo H, Yonetani Y, Mizoguchi H, Hashimoto S, Ozaki A (2000) Process for producing HMG-CoA reductase inhibitor. Patent WO/2000/044886
Ewen KM, Hannemann F, Khatri Y, Perlova O, Kappl R, Krug D, Huttermann J, Muller R, Bernhardt R (2009) Genome mining in Sorangium cellulosum So ce56: identification and characterization of the homologous electron transfer proteins of a myxobacterial cytochrome P450. J Biol Chem 284:28590–28598
Fischer M, Knoll M, Sirim D, Wagner F, Funke S, Pleiss J (2007) The cytochrome P450 engineering database: a navigation and prediction tool for the cytochrome P450 protein family. Bioinformatics 23:2015–2017
Fraatz MA, Berger RG, Zorn H (2009) Nootkatone—a biotechnological challenge. Appl Microbiol Biotechnol 83:35–41
Fujii K, Huennekens FM (1974) Activation of methionine synthetase by a reduced triphosphopyridine nucleotide-dependent flavoprotein system. J Biol Chem 249:6745–6753
Furuya T, Nishi T, Shibata D, Suzuki H, Ohta D, Kino K (2008) Characterization of orphan monooxygenases by rapid substrate screening using FT-ICR mass spectrometry. Chem Biol 15:563–572
Girhard M, Schuster S, Dietrich M, Durre P, Urlacher VB (2007) Cytochrome P450 monooxygenase from Clostridium acetobutylicum: a new alpha-fatty acid hydroxylase. Biochem Biophys Res Commun 362:114–119
Girhard M, Machida K, Itoh M, Schmid RD, Arisawa A, Urlacher VB (2009) Regioselective biooxidation of (+)-valencene by recombinant E. coli expressing CYP109B1 from Bacillus subtilis in a two-liquid-phase system. Microb Cell Fact 8:36
Goni G, Zollner A, Lisurek M, Velazquez-Campoy A, Pinto S, Gomez-Moreno C, Hannemann F, Bernhardt R, Medina M (2009) Cyanobacterial electron carrier proteins as electron donors to CYP106A2 from Bacillus megaterium ATCC 13368. Biochim Biophys Acta 1794:1635–1642
Green AJ, Munro AW, Cheesman MR, Reid GA, von Wachenfeldt C, Chapman SK (2003) Expression, purification and characterisation of a Bacillus subtilis ferredoxin: a potential electron transfer donor to cytochrome P450 BioI. J Inorg Biochem 93:92–99
Gustafsson MC, Roitel O, Marshall KR, Noble MA, Chapman SK, Pessegueiro A, Fulco AJ, Cheesman MR, von Wachenfeldt C, Munro AW (2004) Expression, purification, and characterization of Bacillus subtilis cytochromes P450 CYP102A2 and CYP102A3: flavocytochrome homologues of P450 BM3 from Bacillus megaterium. Biochemistry 43:5474–5487
Hannemann F, Bera AK, Fischer B, Lisurek M, Teuchner K, Bernhardt R (2002) Unfolding and conformational studies on bovine adrenodoxin probed by engineered intrinsic tryptophan fluorescence. Biochemistry 41:11008–11016
Hannemann F, Virus C, Bernhardt R (2006) Design of an Escherichia coli system for whole cell mediated steroid synthesis and molecular evolution of steroid hydroxylases. J Biotechnol 124:172–181
Hannemann F, Bichet A, Ewen KM, Bernhardt R (2007) Cytochrome P450 systems—biological variations of electron transport chains. Biochim Biophys Acta 1770:330–344
Huang JJ, Kimura T (1973) Studies on adrenal steroid hydroxylases. Oxidation–reduction properties of adrenal iron–sulfur protein (adrenodoxin). Biochemistry 12:406–409
Jenkins CM, Waterman MR (1998) NADPH-flavodoxin reductase and flavodoxin from Escherichia coli: characteristics as a soluble microsomal P450 reductase. Biochemistry 37:6106–6113
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Caldwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, Danchin A et al (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249–256
Lawson RJ, Leys D, Sutcliffe MJ, Kemp CA, Cheesman MR, Smith SJ, Clarkson J, Smith WE, Haq I, Perkins JB, Munro AW (2004a) Thermodynamic and biophysical characterization of cytochrome P450 BioI from Bacillus subtilis. Biochemistry 43:12410–12426
Lawson RJ, von Wachenfeldt C, Haq I, Perkins J, Munro AW (2004b) Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: biophysical properties and interactions with cytochrome P450 BioI. Biochemistry 43:12390–12409
Mandai T, Fujiwara S, Imaoka S (2009) A novel electron transport system for thermostable CYP175A1 from Thermus thermophilus HB27. Febs J 276:2416–2429
Matsuzaki F, Wariishi H (2004) Functional diversity of cytochrome P450s of the white-rot fungus Phanerochaete chrysosporium. Biochem Biophys Res Commun 324:387–393
Maurer S, Urlacher V, Schulze H, Schmid RD (2003) Immobilisation of P450 BM-3 and an NADP+ cofactor recycling system: towards a technical application of heme-containing monooxygenases in fine chemical synthesis. Adv Synth Catal 345:802–810
McIver L, Leadbeater C, Campopiano DJ, Baxter RL, Daff SN, Chapman SK, Munro AW (1998) Characterisation of flavodoxin NADP+ oxidoreductase and flavodoxin; key components of electron transfer in Escherichia coli. Eur J Biochem 257:577–585
Meyer K (2002) Colorful antioxidants—carotenoids: significance and technical syntheses. Chem Unserer Zeit 36:178–192
Miura Y, Fulco AJ (1975) Omega-1, Omega-2 and Omega-3 hydroxylation of long-chain fatty acids, amides and alcohols by a soluble enzyme system from Bacillus megaterium. Biochim Biophys Acta 388:305–317
Nelson DR (2006) Cytochrome P450 nomenclature, 2004. Methods Mol Biol 320:1–10
Omura T, Sato R (1964a) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Omura T, Sato R (1964b) The carbon monoxide-binding pigment of liver microsomes. II. Solubilization, purification, and properties. J Biol Chem 239:2379–2385
Purdy MM, Koo LS, Ortiz de Montellano PR, Klinman JP (2004) Steady-state kinetic investigation of cytochrome P450cam: interaction with redox partners and reaction with molecular oxygen. Biochemistry 43:271–281
Sagara Y, Wada A, Takata Y, Waterman MR, Sekimizu K, Horiuchi T (1993) Direct expression of adrenodoxin reductase in Escherichia coli and the functional characterization. Biol Pharm Bull 16:627–630
Sambrook J, Russell D (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Schenkman JB, Sligar SG, Cinti DL (1981) Substrate interaction with cytochrome P-450. Pharmacol Ther 12:43–71
Sevrioukova I, Truan G, Peterson JA (1996) The flavoprotein domain of P450BM-3: expression, purification, and properties of the flavin adenine dinucleotide- and flavin mononucleotide-binding subdomains. Biochemistry 35:7528–7535
Sirim D, Wagner F, Lisitsa A, Pleiss J (2009) The cytochrome P450 engineering database: integration of biochemical properties. BMC Biochem 10:27
Sowden RJ, Yasmin S, Rees NH, Bell SG, Wong LL (2005) Biotransformation of the sesquiterpene (+)-valencene by cytochrome P450cam and P450BM-3. Org Biomol Chem 3:57–64
Uhlmann H, Kraft R, Bernhardt R (1994) C-terminal region of adrenodoxin affects its structural integrity and determines differences in its electron transfer function to cytochrome P-450. J Biol Chem 269:22557–22564
Urlacher VB, Makhsumkhanov A, Schmid RD (2006) Biotransformation of beta-ionone by engineered cytochrome P450 BM-3. Appl Microbiol Biotechnol 70:53–59
Virus C, Bernhardt R (2008) Molecular evolution of a steroid hydroxylating cytochrome P450 using a versatile steroid detection system for screening. Lipids 43:1133–1141
Virus C, Lisurek M, Simgen B, Hannemann F, Bernhardt R (2006) Function and engineering of the 15beta-hydroxylase CYP106A2. Biochem Soc Trans 34:1215–1218
Wang ZQ, Lawson RJ, Buddha MR, Wei CC, Crane BR, Munro AW, Stuehr DJ (2007) Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase. J Biol Chem 282:2196–2202
Acknowledgments
We wish to thank Kyoko Momoi, Sumire Honda Malca, and Svetlana Tihovsky (Universitaet Stuttgart) for their help in preparation of Fdx and cloning of FdR, YkuN, and YkuP. We are also thankful to Wolfgang Reinle for expression and purification of Adx and AdR. MG, TK, and VBU acknowledge the support of this work by Deutsche Forschungsgemeinschaft (SFB706) and Ministerium für Wissenschaft, Forschung und Kunst Baden-Württemberg.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Electronic supplementary material (DOC 223 kb)
Rights and permissions
About this article
Cite this article
Girhard, M., Klaus, T., Khatri, Y. et al. Characterization of the versatile monooxygenase CYP109B1 from Bacillus subtilis . Appl Microbiol Biotechnol 87, 595–607 (2010). https://doi.org/10.1007/s00253-010-2472-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2472-z