Abstract
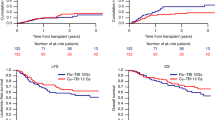
Standard myeloablative conditioning regimens for children with acute lymphoblastic leukaemia are based on total body irradiation (TBI). However, TBI causes profound short-term and long-term side effects, provoking the necessity for alternative regimens. Treosulfan combines a potent immunosuppressive and antileukaemic effect with myeloablative activity and low toxicity profile. We retrospectively studied toxicity and outcome of 71 paediatric patients with acute lymphoblastic leukaemia (ALL) undergoing haematopoietic stem cell transplantation (HSCT) following treosulfan-based conditioning aiming to identify risk factors for treatment failure and dose-depending outcome differences. Early regimen-related toxicity was low. No case of veno-occlusive disease was reported. There was no association of toxicity with age or number of HSCT. Event-free survival (EFS) of infants was significantly better compared to older children. Overall survival (OS) at 3 years was 51 % and not significantly influenced by number of HSCT (first HSCT 54 %, ≥second HSCT 44 %, p = 0.71). In multivariate analysis, OS and EFS were significantly worse for patients transplanted without complete remission (p = 0.04 and 0.004). Treatment-related mortality was low at 14 %. We conclude that treosulfan-based conditioning is a safe and efficacious approach for paediatric ALL.


Similar content being viewed by others
References
Peters C, Schrauder A, Schrappe M et al (2005) Allogeneic haematopoietic stem cell transplantation in children with acute lymphoblastic leukaemia: the BFM/IBFM/EBMT concepts. Bone Marrow Transplant 35(Suppl 1):S9–11
Schrappe M, Reiter A, Zimmermann M et al (2000) Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster. Leukemia 14:2205–2222
Pulsipher MA, Peters C, Pui CH (2011) High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant 17:S137–148
Eckert C, Henze G, Seeger K et al (2013) Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol 31:2736–2742
Schrauder A, von Stackelberg A, Schrappe M, Cornish J, Peters C (2008) Allogeneic hematopoietic SCT in children with all: current concepts of ongoing prospective SCT trials. Bone Marrow Transplant 41(Suppl 2):S71–74
Balduzzi A, Valsecchi MG, Uderzo C et al (2005) Chemotherapy versus allogeneic transplantation for very-high-risk childhood acute lymphoblastic leukaemia in first complete remission: comparison by genetic randomisation in an international prospective study. Lancet 366:635–642
Balduzzi A, Valsecchi MG, Silvestri D et al (2002) Transplant-related toxicity and mortality: an AIEOP prospective study in 636 pediatric patients transplanted for acute leukemia. Bone Marrow Transplant 29:93–100
Linsenmeier C, Thoennessen D, Negretti L et al (2010) Total body irradiation (TBI) in pediatric patients. a single-center experience after 30 years of low-dose rate irradiation. Strahlenther Onkol 186:614–620
Ricardi U, Filippi AR, Biasin E et al (2009) Late toxicity in children undergoing hematopoietic stem cell transplantation with TBI-containing conditioning regimens for hematological malignancies. Strahlenther Onkol 185(Suppl 2):17–20
Bresters D, van Gils IC, Kollen WJ et al (2010) High burden of late effects after haematopoietic stem cell transplantation in childhood: a single-centre study. Bone Marrow Transplant 45:79–85
Marks DI, Forman SJ, Blume KG et al (2006) A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol Blood Marrow Transplant 12:438–453
Davies SM, Ramsay NK, Klein JP et al (2000) Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J Clin Oncol 18:340–347
Baron F, Storb R, Storer BE et al (2006) Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. J Clin Oncol 24:4150–4157
Pulsipher MA, Boucher KM, Wall D et al (2009) Reduced-intensity allogeneic transplantation in pediatric patients ineligible for myeloablative therapy: results of the pediatric blood and marrow transplant consortium study ONC0313. Blood 114:1429–1436
Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan A, Simms S (2003) Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a pediatric blood and marrow transplant consortium study. Bone Marrow Transplant 32:543–548
Del Toro G, Satwani P, Harrison L et al (2004) A pilot study of reduced intensity conditioning and allogeneic stem cell transplantation from unrelated cord blood and matched family donors in children and adolescent recipients. Bone Marrow Transplant 33:613–622
Scheulen ME, Hilger RA, Oberhoff C et al (2000) Clinical phase I dose escalation and pharmacokinetic study of high-dose chemotherapy with treosulfan and autologous peripheral blood stem cell transplantation in patients with advanced malignancies. Clin Cancer Res 6:4209–4216
Glowka FK, Karazniewicz-Lada M, Grund G, Wrobel T, Wachowiak J (2008) Pharmacokinetics of high-dose i.v. treosulfan in children undergoing treosulfan-based preparative regimen for allogeneic haematopoietic SCT. Bone Marrow Transplant 42(2):S67–70
Wachowiak J, Sykora KW, Cornish J et al (2011) Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT pediatric diseases working party. Bone Marrow Transplant 46:1510–1518
Fichtner I, Becker M, Baumgart J (2003) Antileukaemic activity of treosulfan in xenografted human acute lymphoblastic leukaemias (ALL). Eur J Cancer 39:801–807
van Pel M, van Breugel DW, Vos W, Ploemacher RE, Boog CJ (2003) Towards a myeloablative regimen with clinical potential: I. Treosulfan conditioning and bone marrow transplantation allow induction of donor-specific tolerance for skin grafts across full MHC barriers. Bone Marrow Transplant 32:15–22
Poetschger U, Sykora K-W, Zecca M et al (2012) Meta-analysis on treosulfan for conditioning in children and adolescents before hematopoietic stem cell transplantation. Bone Marrow Transplant 47:S37
Glucksberg H, Storb R, Fefer A et al (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation 18:295–304
Klein JP, Rizzo JD, Zhang MJ, Keiding N (2001) Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part 2: regression modeling. Bone Marrow Transplant 28:1001–1011
Klein JP, Rizzo JD, Zhang MJ, Keiding N (2001) Statistical methods for the analysis and presentation of the results of bone marrow transplants. Part I: unadjusted analysis. Bone Marrow Transplant 28:909–915
Fine JG, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 9:496–509
Gray RJ (1988) A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
Hosmer D, Lemeshow S (2000) Applied logistic regression. Wiley, New York, 2nd edition
Levy JM. Tello T. Giller R. et al. Late effects of total body irradiation and hematopoietic stem cell transplant in children under 3 years of age. Pediatr Blood Cancer 2012.
Vassal G, Deroussent A, Hartmann O et al (1990) Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res 50:6203–6207
Carpenter PA, Marshall GM, Giri N, Vowels MR, Russell SJ (1996) Allogeneic bone marrow transplantation for children with acute lymphoblastic leukemia conditioned with busulfan, cyclophosphamide and melphalan. Bone Marrow Transplant 18:489–494
Eom KS, Shin SH, Yoon JH et al (2013) Comparable long-term outcomes after reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high-risk acute lymphoblastic leukemia in complete remission. Am J Hematol 88:634–641
Satwani P, Morris E, Bradley MB, Bhatia M, van de Ven C, Cairo MS (2008) Reduced intensity and non-myeloablative allogeneic stem cell transplantation in children and adolescents with malignant and non-malignant diseases. Pediatr Blood Cancer 50:1–8
Bartelink IH, Bredius RG, Belitser SV et al (2009) Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant 15:231–241
Ansari M. Theoret Y. Rezgui MA. et al. Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematopoietic stem cell transplantation. Ther Drug Monit 2013
Munkelt D, Koehl U, Kloess S et al (2008) Cytotoxic effects of treosulfan and busulfan against leukemic cells of pediatric patients. Cancer Chemother Pharmacol 62:821–830
Hilgendorf I, Wolff D, Gromke T et al (2011) Retrospective analysis of treosulfan-based conditioning in comparison with standard conditioning in patients with myelodysplastic syndrome. Bone Marrow Transplant 46:502–509
Nemecek ER, Guthrie KA, Sorror ML et al (2011) Conditioning with treosulfan and fludarabine followed by allogeneic hematopoietic cell transplantation for high-risk hematologic malignancies. Biol Blood Marrow Transplant 17:341–350
Danylesko I, Shimoni A, Nagler A (2012) Treosulfan-based conditioning before hematopoietic SCT: more than a BU look-alike. Bone Marrow Transplant 47:5–14
Casper J, Wolff D, Knauf W et al (2010) Allogeneic hematopoietic stem-cell transplantation in patients with hematologic malignancies after dose-escalated treosulfan/fludarabine conditioning. J Clin Oncol 28:3344–3351
Corker E, Astwood E, Williams J, Vora A (2012) Treosulphan-based radiation-free myeloablative conditioning for allogeneic transplant in infant acute lymphoblastic leukaemia. Br J Haematol 159:104–106
Slatter MA, Rao K, Amrolia P et al (2011) Treosulfan-based conditioning regimens for hematopoietic stem cell transplantation in children with primary immunodeficiency: United Kingdom experience. Blood 117:4367–4375
Beier R, Schulz A, Honig M et al (2013) Long-term follow-up of children conditioned with Treosulfan: German and Austrian experience. Bone Marrow Transplant 48:491–501
Bernardo ME, Piras E, Vacca A et al (2012) Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood 120:473–476
Law J, Cowan MJ, Dvorak CC et al (2012) Busulfan, fludarabine, and alemtuzumab as a reduced toxicity regimen for children with malignant and nonmalignant diseases improves engraftment and graft-versus-host disease without delaying immune reconstitution. Biol Blood Marrow Transplant 18:1656–1663
Heli U, Eeva J, Anne N, Tapani R, Liisa V (2012) Low incidence and severity of oral mucositis in allogeneic stem cell transplantation after conditioning with treosulfan and fludarabine. Eur J Haematol 88:87–88
Greystoke B, Bonanomi S, Carr TF et al (2008) Treosulfan-containing regimens achieve high rates of engraftment associated with low transplant morbidity and mortality in children with non-malignant disease and significant co-morbidities. Br J Haematol 142:257–262
Kato M, Takahashi Y, Tomizawa D et al (2013) Comparison of intravenous with oral busulfan in allogeneic hematopoietic stem cell transplantation with myeloablative conditioning regimens for pediatric acute leukemia. Biol Blood Marrow Transplant 19:1690–1694
Vettenranta K (2008) Current European practice in pediatric myeloablative conditioning. Bone Marrow Transplant 41(Suppl 2):S14–17
Bartelink IH. van Reij EM. Gerhardt CE. et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant 2013.
Kato M, Horikoshi Y, Okamoto Y et al (2012) Second allogeneic hematopoietic SCT for relapsed all in children. Bone Marrow Transplant 47:1307–1311
Mann G, Attarbaschi A, Schrappe M et al (2010) Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood 116:2644–2650
Pui C-H, Gaynon PS, Boyett JM et al (2002) Outcome of treatment in childhood acute lymphoblastic leukaemia with rearrangements of the 11q23 chromosomal region. Lancet 359:1909–1915
Pieters R, Schrappe M, De Lorenzo P et al (2007) A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet 370:240–250
Cutting R, Mirelman A, Vora A (2008) Treosulphan as an alternative to busulphan for myeloablative conditioning in paediatric allogeneic transplantation. Br J Haematol 143:748–751
Knechtli CJ, Goulden NJ, Hancock JP et al (1998) Minimal residual disease status before allogeneic bone marrow transplantation is an important determinant of successful outcome for children and adolescents with acute lymphoblastic leukemia. Blood 92:4072–4079
Duerst RE, Horan JT, Liesveld JL et al (2000) Allogeneic bone marrow transplantation for children with acute leukemia: cytoreduction with fractionated total body irradiation, high-dose etoposide and cyclophosphamide. Bone Marrow Transplant 25:489–494
Balis FM, Poplack DG (1989) Central nervous system pharmacology of antileukemic drugs. Am J Pediatr Hematol Oncol 11:74–86
Acknowledgments
We thank Arnaud Dalassier for data correction and management.
Conflict of interest
The EBMT received an unrestricted grant for the retrospective data collection and analysis from medac GmbH. CP received travel grants and study support from medac, Pierre Fabre, Sanofi and Novartis. HB received a travel grant from Pierre Fabre. KWS received travel grants and study support from medac.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Boztug, H., Zecca, M., Sykora, KW. et al. Treosulfan-based conditioning regimens for allogeneic HSCT in children with acute lymphoblastic leukaemia. Ann Hematol 94, 297–306 (2015). https://doi.org/10.1007/s00277-014-2196-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2196-8