Abstract
Background
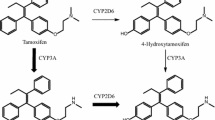
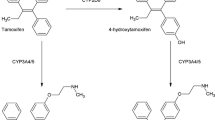
Reduced CYP2D6 metabolism and low Z-endoxifen (ENDX) concentrations may increase the risk of breast cancer recurrence in tamoxifen (TAM)-treated women. Little is known regarding the differences between TAM and ENDX murine pharmacokinetics or the effect of administration route on plasma concentrations of each drug.
Methods
The pharmacokinetics of TAM and ENDX were characterized in female mice.
Results
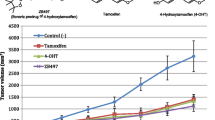
For subcutaneous [s.c.] and oral TAM (4, 10 and 20 mg/kg), TAM AUC increased in a linear manner, but concentrations of the active metabolites [ENDX and 4-hydroxytamoxifen (4HT)] remained low. For oral TAM (20 mg), 4HT concentrations were tenfold greater (>25 ng/ml) than achievable in TAM-treated humans. Both oral (10–200 mg/kg) and s.c. (2.5–25 mg/kg) ENDX·HCl resulted in a greater than dose-proportional increase in AUC, with eightfold greater ENDX concentrations than an equivalent TAM dose. ENDX accumulated in plasma after 5-day dosing of 25 or 100 mg/kg ENDX·HCl and exceeded target concentrations of 0.1 and 1.0 μM, respectively, by twofold to fourfold.
Conclusions
In murine models, oral ENDX yields substantially higher ENDX concentrations, compared to TAM. The low 4HT and ENDX concentrations observed in mice receiving s.c. TAM mirror the TAM pharmacokinetics in humans with impaired CYP2D6 metabolism. These data support the ongoing development of ENDX as a novel agent for the endocrine treatment of ER-positive breast cancer.


Similar content being viewed by others
Abbreviations
- CYP2D6:
-
Cytochrome P450
- ENDX:
-
Endoxifen (4-hydroxy-N-desmethyl tamoxifen)
- TAM:
-
Tamoxifen
- 4HT:
-
4-Hydroxytamoxifen
- NDMT:
-
N-Desmethyltamoxifen
- ER:
-
Estrogen receptor
- s.c.:
-
Subcutaneous
- i.v.:
-
Intravenous
- HPLC:
-
High-pressure liquid chromatography
- ACN:
-
Acetonitrile
- KH2PO4 :
-
Monopotassium phosphate
- PEG:
-
Polyethylene glycol
- CMC:
-
Carboxymethylcellulose
- CPDA:
-
Citrate/phosphate/dextrose/adenine anticoagulant solution
- AUC:
-
Area under the plasma concentration–time curve
- C max :
-
Maximum plasma concentration
- T max :
-
Time that the maximum plasma concentration was achieved
- Vz:
-
Volume of distribution
- Clp:
-
Plasma clearance
References
Lonning PE, Lien EA, Lundgren S, Kvinnsland S (1992) Clinical pharmacokinetics of endocrine agents used in advanced breast cancer. Clin Pharmacokinet 22:327–358
Desta Z, Ward BA, Soukhova NV, Flockhart DA (2004) Comprehensive evaluation of tamoxifen sequential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A and CYP2D6. J Pharmacol Exp Ther 310:1062–1075
Borgna JL, Rochefort H (1981) Hydroxylated metabolites of tamoxifen are formed in vivo and bound to estrogen receptor in target tissues. J Biol Chem 256:859–868
Robertson DW, Katzenellenbogen JA, Long DJ, Rorke EA, Katzenellenbogen BS (1982) Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem 16:1–13
Jordan VC (1982) Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance. Breast Cancer Res Treat 2:123–138
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478
Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC (2009) The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res 69:1722–1727
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Jin Y, Desta Z, Stearns V, Ward B, Ho H, Lee KH, Skaar T, Storniolo AM, Li L, Araba A, Blanchard R, Nguyen A, Ullmer L, Hayden J, Lemler S, Weinshilboum RM, Rae JM, Hayes DF, Flockhart DA (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Borges S, Desta Z, Li L, Skaar TC, Ward BA, Nguyen A, Jin Y, Storniolo AM, Nikoloff DM, Wu L, Hillman G, Hayes DF, Stearns V, Flockhart DA (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80:61–74
Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE, Kisanga ER, Mellgren G, Steen VM, Lien EA (2008) Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol 19:56–61
Stearns V, Beebe KL, Iyengar M, Dube E (2003) Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 289:2827–2834
Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP, Salter J, Sestak I, Cuzick J, Dowsett M (2012) CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst 104:452–460
Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, Dell’orto P, Biasi MO, Thurlimann B, Lyng MB, Ditzel HJ, Neven P, Debled M, Maibach R, Price KN, Gelber RD, Coates AS, Goldhirsch A, Rae JM, Viale G (2012) CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst 104:441–451
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 89:718–725
Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL, Kuffel M, Jakesz R, Rudas M, Greil R, Dietze O, Lang A, Offner F, Reynolds CA, Weinshilboum RM, Ames MM, Ingle JN (2013) CYP2D6 metabolism and patient outcome in the austrian breast and colorectal cancer study group trial (abcsg) 8. Clin Cancer Res 19:500–507
Karle J, Bolbrinker J, Vogl S, Kreutz R, Denkert C, Eucker J, Wischnewsky M, Possinger K, Regierer AC (2013) Influence of CYP2D6-genotype on tamoxifen efficacy in advanced breast cancer. Breast Cancer Res Treat 139:553–560
Lee KH, Ward BA, Desta Z, Flockhart DA, Jones DR (2003) Quantification of tamoxifen and three metabolites in plasma by high-performance liquid chromatography with fluorescence detection: application to a clinical trial. J Chromatogr 791:245–253
Schiff R, Reddy P, Ahotupa M, Coronado-Heinsohn E, Grim M, Hilsenbeck SG, Lawrence R, Deneke S, Herrera R, Chamness GC, Fuqua SA, Brown PH, Osborne CK (2000) Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J Natl Cancer Inst 92:1926–1934
Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, Fasching PA, Fehm T, Eichelbaum M, Schwab M, Brauch H (2011) Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther 89:708–717
McLaughlin LA, Dickmann LJ, Wolf CR, Henderson CJ (2008) Functional expression and comparative characterization of nine murine cytochromes P450 by fluorescent inhibition screening. Drug Metab Dispos 36:1322–1331
Lofgren S, Hagbjork AL, Ekman S, Fransson-Steen R, Terelius Y (2004) Metabolism of human cytochrome P450 marker substrates in mouse: a strain and gender comparison. Xenobiotica 34:811–834
Jordan VC, Allen KE (1980) Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer 16:239–251
Robinson SP, Langan-Fahey SM, Jordan VC (1989) Implications of tamoxifen metabolism in the athymic mouse for the study of antitumor effects upon human breast cancer xenografts. Eur J Cancer Clin Oncol 25:1769–1776
Masubuchi Y, Iwasa T, Hosokawa S, Suzuki T, Horie T, Imaoka S, Funae Y, Narimatsu S (1997) Selective deficiency of debrisoquine 4-hydroxylase activity in mouse liver microsomes. J Pharmacol Exp Ther 282:1435–1441
Ahmad A, Ali SM, Ahmad MU, Sheikh S, Ahmad I (2010) Orally administered endoxifen is a new therapeutic agent for breast cancer. Breast Cancer Res Treat 122:579–584
Gong IY, Teft WA, Ly J, Chen YH, Alicke B, Kim RB, Choo EF (2013) Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat 139:61–69
Zhao XJ, Jones DR, Wang YH, Grimm SW, Hall SD (2002) Reversible and irreversible inhibition of CYP3A enzymes by tamoxifen and metabolites. Xenobiotica 32:863–878
Bekaii-Saab TS, Perloff MD, Weemhoff JL, Greenblatt DJ, von Moltke LL (2004) Interactions of tamoxifen, N-desmethyltamoxifen and 4-hydroxytamoxifen with P-glycoprotein and CYP3A. Biopharm Drug Dispos 25:283–289
Shin SC, Choi JS, Li X (2006) Enhanced bioavailability of tamoxifen after oral administration of tamoxifen with quercetin in rats. Int J Pharm 313:144–149
Teft WA, Mansell SE, Kim RB (2011) Endoxifen, the active metabolite of tamoxifen, is a substrate of the efflux transporter p-glycoprotein (multidrug resistance 1). Drug Metab Dispos 39:558–562
Acknowledgments
Supported in part by 1R01CA133049-01 (MPG, MMA), the Mayo Comprehensive Cancer Center Grant (CA15083; MMA, JMR, VS), and the Mayo Clinic Breast Cancer SPORE (CA 116201; MMA, JMR, MPG, PH, XH).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Joel M. Reid and Matthew P. Goetz have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Reid, J.M., Goetz, M.P., Buhrow, S.A. et al. Pharmacokinetics of endoxifen and tamoxifen in female mice: implications for comparative in vivo activity studies. Cancer Chemother Pharmacol 74, 1271–1278 (2014). https://doi.org/10.1007/s00280-014-2605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2605-7