Abstract
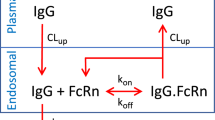
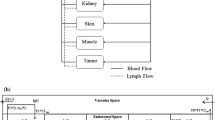
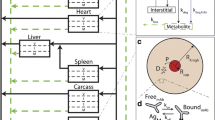
We constructed a novel physiologically-based pharmacokinetic (PBPK) model for predicting interactions between the neonatal Fc receptor (FcRn) and anti-carcinoembryonic antigen (CEA) monoclonal antibodies (mAbs) with varying affinity for FcRn. Our new model, an integration and extension of several previously published models, includes aspects of mAb-FcRn dynamics within intracellular compartments not represented in previous PBPK models. We added mechanistic structure that details internalization of class G immunoglobulins by endothelial cells, subsequent FcRn binding, recycling into plasma of FcRn-bound IgG and degradation of free endosomal IgG. Degradation in liver is explicitly represented along with the FcRn submodel in skin and muscle. A variable tumor mass submodel is also included, used to estimate the growth of an avascular, necrotic tumor core, providing a more realistic picture of mAb uptake by tumor. We fitted the new multiscale model to published anti-CEA mAb biodistribution data, i.e. concentration-time profiles in tumor and various healthy tissues in mice, providing new estimates of mAb-FcRn related kinetic parameters. The model was further validated by successful prediction of F(ab′)2 mAb fragment biodistribution, providing additional evidence of its potential value in optimizing intact mAb and mAb fragment dosing for clinical imaging and immunotherapy applications.
Similar content being viewed by others
References
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control AC-19:716–723, 1974.
Barrett, P. H., B. M. Bell, C. Cobelli, H. Golde, A. Schumitzky, P. Vicini, and D. M. Foster. SAAM II: Simulation, analysis, and modeling software for tracer and pharmacokinetic studies. Metabolism 47:484–492, 1998.
Baxter, L. T., H. Zhu, D. G. Mackensen, W. F. Butler, and R. K. Jain. Biodistribution of monoclonal antibodies: Scale-up from mouse to human using a physiologically based pharmacokinetic model. Cancer Res. 55:4611–4622, 1995.
Baxter, L. T., H. Zhu, D. G. Mackensen, and R. K. Jain. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 54:1517–1528, 1994.
Bell, B. M., J. V. Burke, and A. Schumitzky. A relative weighting method for estimating parameters and variances in multiple data sets. Comput. Stat. Data. An. 22:119–135, 1996.
Berk, D. A., F. Yuan, M. Leunig, and R. K. Jain. Direct in vivo measurement of targeted binding in a human tumor xenograft. Proc. Natl. Acad. Sci. U.S.A. 94:1785–1790, 1997.
Borvak, J., J. Richardson, C. Medesan, F. Antohe, C. Radu, M. Simionescu, V. Ghetie, and E. S. Ward. Functional expression of the MHC class I-related receptor, FcRn, in endothelial cells of mice. Int. Immunol. 10:1289–1298, 1998.
Brambell, F. W., W. A. Hemmings, and I. G. Morris. A theoretical model of gamma-globulin catabolism. Nature 203:1352–1354, 1964.
Brown, R. P., M. D. Delp, S. L. Lindstedt, L. R. Rhomberg, and R. P. Beliles. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 13:407–484, 1997.
Burmeister, W. P., A. H. Huber, and P. J. Bjorkman. Crystal structure of the complex of rat neonatal Fc receptor with Fc. Nature 372:379–383, 1994.
Carter, P. Improving the efficacy of antibody-based cancer therapies. Nat. Rev. Cancer 1:118–129, 2001.
Covell, D. G., J. Barbet, O. D. Holton, C. D. Black, R. J. Parker, and J. N. Weinstein. Pharmacokinetics of monoclonal immunoglobulin G1, F(ab′)2, and Fab’ in mice. Cancer Res. 46:3969–3978, 1986.
Dedrick, R. L. Animal scale-up. J. Pharmacokinet. Biopharm. 1:435–461, 1973.
Dias, S., K. Hattori, B. Heissig, Z. Zhu, Y. Wu, L. Witte, D. J. Hicklin, M. Tateno, P. Bohlen, M. A. Moore, and S. Rafii. Inhibition of both paracrine and autocrine VEGF/VEGFR-2 signaling pathways is essential to induce long-term remission of xenotransplanted human leukemias. Proc. Natl. Acad. Sci. U.S.A. 98:10857–10862, 2001.
Fahey, J. L., and A. G. Robinson. Factors controlling serum gamma-globulin concentration. J. Exp. Med. 118:845–868, 1963.
Fukumoto, T., M. R. Brandon. Importance of the liver in immunoglobulin catabolism. Res. Vet. Sci. 32:62–69, 1982.
Gerlowski, L. E., and R. K. Jain. Physiologically based pharmacokinetic modeling: principles and applications. J. Pharm. Sci. 72:1103–1127, 1983.
Ghetie, V., J. G. Hubbard, J. K. Kim, M. F. Tsen, Y. Lee, and E. S. Ward. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur. J. Immunol. 26:690–696, 1996.
Ghetie, V., and E. S. Ward. Transcytosis and catabolism of antibody. Immunol. Res. 25:97–113, 2002.
Green, A. J., C. J. Johnson, K. L. Adamson, and R. H. Begent. Mathematical model of antibody targeting: important parameters defined using clinical data. Phys. Med. Biol. 46:1679–1693, 2001.
Hansen, R. J., and J. P. Balthasar. Pharmacokinetic/pharmacodynamic modeling of the effects of intravenous immunoglobulin on the disposition of antiplatelet antibodies in a rat model of immune thrombocytopenia. J. Pharm. Sci. 92:1206–1215, 2003.
Hefta, L. J., M. Neumaier, and J. E. Shively. Kinetic and affinity constants of epitope specific anti-carcinoembryonic antigen (CEA) monoclonal antibodies for CEA and engineered CEA domain constructs. Immunotechnology 4:49–57, 1998.
Israel, E. J., V. K. Patel, S. F. Taylor, A. Marshak-Rothstein, and N. E. Simister. Requirement for a beta 2-microglobulin-associated Fc receptor for acquisition of maternal IgG by fetal and neonatal mice. J. Immunol. 154:6246–6251, 1995.
Israel, E. J., D. F. Wilsker, K. C. Hayes, D. Schoenfeld, and N. E. Simister. Increased clearance of IgG in mice that lack beta 2-microglobulin: possible protective role of FcRn. Immunology 89:573–578, 1996.
Jain, R. K. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J. Natl. Cancer Inst. 81:570–576, 1989.
Jones, E. A., and T. A. Waldmann. The mechanism of intestinal uptake and transcellular transport of IgG in the neonatal rat. J. Clin. Invest. 51:2916–2927, 1972.
Junghans, R. P., and C. L. Anderson. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc. Natl. Acad. Sci. U.S.A. 93:5512–5516, 1996.
Keener, J. P., and J. Sneyd. Mathematical Physiology, 8th ed. New York: Springer, 1998, p. 766.
Kim, J. K., M. Firan, C. G. Radu, C. H. Kim, V. Ghetie, and E. S. Ward. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol. 29:2819–2825, 1999.
Ludwig, D. L., D. S. Pereira, Z. Zhu, D. J. Hicklin, and P. Bohlen. Monoclonal antibody therapeutics and apoptosis. Oncogene 22:9097–9106, 2003.
Maloney, D. G., A. J. Grillo-Lopez, D. J. Bodkin, C. A. White, T. M. Liles, I. Royston, C. Varns, J. Rosenberg, and R. Levy. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J. Clin. Oncol. 15:3266–3274, 1997.
Mink, J. G., J. Radl, P. van den Berg, J. J. Haaijman, M. J. van Zwieten, and R. Benner. Serum immunoglobulins in nude mice and their heterozygous littermates during ageing. Immunology 40:539–545, 1980.
Nestorov, I. Whole body pharmacokinetic models. Clin. Pharmacokinet. 42:883–908, 2003.
Ober, R. J., C. Martinez, C. Vaccaro, J. Zhou, and E. S. Ward. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J. Immunol. 172:2021–2029, 2004.
Rastetter, W., A. Molina, and C. A. White. Rituximab: expanding role in therapy for lymphomas and autoimmune diseases. Annu. Rev. Med. 55:477–503, 2004.
Riggs, D. S. The Mathematical Approach to Physiological Problems; A Critical Primer, 14th ed. Baltimore: Williams & Wilkins Co., 1963, p. 445.
Riley, J. K., and M. X. Sliwkowski. CD20: A gene in search of a function. Semin. Oncol. 27:17–24, 2000.
Rippe, B., and B. Haraldsson. Transport of macromolecules across microvascular walls: The two-pore theory. Physiol. Rev. 74:163–219, 1994.
Rodewald, R., and J. P. Kraehenbuhl. Receptor-mediated transport of IgG. J. Cell. Biol. 99:159s–164s, 1984.
Roopenian, D. C., G. J. Christianson, T. J. Sproule, A. C. Brown, S. Akilesh, N. Jung, S. Petkova, L. Avanessian, E. Y. Choi, D. J. Shaffer, P. A. Eden, and C. L. Anderson. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 170:3528–3533, 2003.
Rubin, I., and Y. Yarden. The basic biology of HER2. Ann. Oncol. 12 Suppl 1:S3–S8, 2001.
Rygaard, K., and M. Spang-Thomsen. Quantitation and gompertzian analysis of tumor growth. Breast Cancer Res. Treat. 46:303–312, 1997.
Seber, G. A. F., and C. J. Wild. Nonlinear regression, 20th ed., Hoboken, NJ: Wiley-Interscience, 2003, p. 768.
Sharkey, R. M., A. Natale, D. M. Goldenberg, M. J. Mattes. Rapid blood clearance of immunoglobulin G2a and immunoglobulin G2b in nude mice. Cancer Res. 51:3102–3107, 1991.
Simister, N. E. Placental transport of immunoglobulin G. Vaccine 21:3365–3369, 2003.
Theil, F. P., T. W. Guentert, S. Haddad, and P. Poulin. Utility of physiologically based pharmacokinetic models to drug development and rational drug discovery candidate selection. Toxicol. Lett. 138:29–49, 2003.
Vaughn, D. E., and P. J. Bjorkman. High-affinity binding of the neonatal Fc receptor to its IgG ligand requires receptor immobilization. Biochemistry 36:9374–9380, 1997.
Waldmann, T. A., and W. Strober. Metabolism of immunoglobulins. Prog. Allergy 13:1–110, 1969.
Williams, L. E., R. B. Duda, R. T. Proffitt, B. G. Beatty, J. D. Beatty, J. Y. Wong, J. E. Shively, and R. J. Paxton. Tumor uptake as a function of tumor mass: A mathematic model. J. Nucl. Med. 29:103–109, 1988.
Williams, L. E., A. M. Wu, P. J. Yazaki, A. Liu, A. A. Raubitschek, J. E. Shively, and J. Y. Wong. Numerical selection of optimal tumor imaging agents with application to engineered antibodies. Cancer Biother. Radiopharm. 16:25–35, 2001.
Zhou, Y. Choice of designs and doses for early phase trials. Fundam. Clin. Pharmacol. 18:373–378, 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10439-011-0373-7.
Rights and permissions
About this article
Cite this article
Ferl, G.Z., Wu, A.M. & DiStefano, J.J. A Predictive Model of Therapeutic Monoclonal Antibody Dynamics and Regulation by the Neonatal Fc Receptor (FcRn). Ann Biomed Eng 33, 1640–1652 (2005). https://doi.org/10.1007/s10439-005-7410-3
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10439-005-7410-3