Abstract
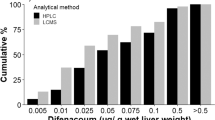
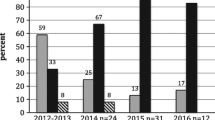
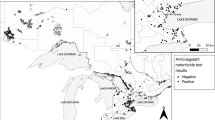
Despite the documented risk of secondary poisoning to non-target species by anticoagulant rodenticides there is no statutory post-approval monitoring of their use in the UK. This paper presents results from two Scottish monitoring schemes for the period 2000–2010; recording rodenticide use on arable farms and the presence of residues in raptor carcasses. More than three quarters of arable farms used anticoagulant rodenticides; predominately the second generation compounds difenacoum and bromadiolone. There was widespread exposure to anticoagulant rodenticides in liver tissues of the raptor species tested and the residues encountered generally reflected agricultural use patterns. As found in other studies, Red Kites (Milvus milvus) appeared to be particularly vulnerable to rodenticide exposure, 70 % of those sampled (n = 114) contained residues and 10 % died as a result of rodenticide ingestion. More unexpectedly, sparrowhawks (Accipiter nisus), which prey almost exclusively on birds, had similar exposure rates to species which prey on rodents. Although, with the exception of kites, confirmed mortality from rodenticides was low, the widespread exposure recorded is concerning. Particularly when coupled with a lack of data about the sub-lethal effects of these compounds. This raises questions regarding whether statutory monitoring of use is needed; both to address whether there are deficiencies in compliance with approval conditions or whether the recommended risk management procedures are themselves adequate to protect non-target wildlife.



Similar content being viewed by others
References
Albert CA, Wilson LK, Mineau P, Trudeau S, Elliott JE (2010) Anticoagulant rodenticides in three owl species from Western Canada, 1988–2003. Arch Environ Contam Toxicol 58:451–459
Anonymous (2007) Difenacoum pesticide fact sheet. Environmental Protection Agency, Seattle, p 34
Berny P, Gaillet JR (2008) Acute poisoning of red kites (Milvus milvus) in France: data from SAGIR network. J Wildl Dis 44:417–426
Berny PJ, Buronfosse T, Buronfosse F, Lamarque F, Lorgue G (1997) Field evidence of secondary poisoning of foxes (Vulpes vulpes) and buzzards (Buteo buteo) by bromadiolone, a 4-year survey. Chemosphere 35:1817–1829
Booth LH, Eason CT, Spurr EB (2001) Literature review of the acute toxicity and persistence of brodifacoum to invertebrates. Sci Conserv 177:1–9
Brakes CR, Smith RH (2005) Exposure of non-target small mammals to rodenticides: short-term effects, recovery and implications for secondary poisoning. J Appl Ecol 42:118–128
Buckle AP, Prescott CV, Ward KJ (1994) Resistance to the first and second generation anticoagulant rodenticides—a new perspective. In: Proceedings of the sixteenth vertebrate pest conference, University of California, Davis, pp 138–144
Carter I, Burn A (2000) Problems with rodenticides: the threat to red kites and other wildlife. Br Wildl 11:192–197
Cox P, Smith RH (1992) Rodenticide ecotoxicology: pre-lethal effects of anticoagulants on rat behaviour. In: Proceedings of the fifteenth vertebrate pest conference, University of Nebraska, Lincoln, pp 65–170
Dowding CV, Shore RF, Worgan R, Baker PJ, Harris S (2010) Accumulation of anticoagulant rodenticides in a non-target insectivore, the European hedgehog (Erinaceus europaeus). Environ Pollut 158:161–166
Dunlevy PA, Campbell EW, Lindsey GW (2000) Broadcast application of a placebo rodenticide bait in a native Hawaiian forest. Int J Biodeter Biodegrad 45:199–208
DuVall MD, Murphy MJ, Ray AC, Reagor JC (1989) Case studies on second-generation anticoagulant rodenticide toxicities in non target species. J Vet Diagn Invest 1:66–68
Eason CT, Murphy E (2001) Recognising and reducing secondary and tertiary risks associated with brodifacoum. In: Johnston JJ (ed) Pesticides and wildlife. American chemical society symposium series 771, pp 157–163
Empson RA, Miskelly CM (1999) The risks, costs and benefits of using brodifacoum to eradicate rats from kapiti Island, New Zealand. N Z J Ecol 23:241–254
Erickson W, Urban D (2004) Potential risks of nine rodenticides to birds and non-target mammals: a comparative approach. Environmental protection agency office of prevention, pesticides and toxic substances, Washington, DC
Fisher PM (2009) Residual concentrations and persistence of the anticoagulant rodenticides brodifacoum and diphacinone in fauna dissertation. Lincoln University, New Zealand
Hughes J (2012) Pesticide usage in Scotland: rodenticides on arable farms 2000 to 2010. Scottish Government Agriculture Food and Rural Communities Directorate, Edinburgh
Hunter K, Sharp EA (1988) Modification to procedures for the determination of chlorophacinone and for multi-residue analysis of rodenticides in animal tissues. J Chromatogr 437:301–305
Joermann G (1998) A review of secondary-poisoning studies with rodenticides. Bull OEPP 28:157–176
Johnston JJ, Pitt WC, Sugihara RT, Eisemann JD, Primus TM, Holmes MJ, Crocker J, Hart A (2005) Probabilistic risk assessment for snails, slugs and endangered honeycreepers in diphacinone rodenticide baited areas on Hawaii, USA. Environ Toxicol Chem 24:1557–1567
Leemus JA, Bravo C, Garcia-Montijano M, Palacín C, Ponce C, Magańa M, Alonso JC (2011) Side effects of rodent control on non-target species: rodenticides increase parasite and pathogen burden in great bustards. Sci Total Environ 409:4729–4734
Meehan AP (1984) Rats and mice: their biology and control. Rentokil Limited, East Grinstead
Morgan DR, Wright GR, Ogilvie SC, Pierce R, Thompson P (1996) Assessment of the environmental impact of brodifacoum during rodent eradication operations in New Zealand. In: Proceedings of the seventeenth vertebrate pest conference, Rhonert Park, California, pp 213–218
Myllymäki A, Pihlava J, Tuuri H (1999) Predicting the exposure and risk to predators and scavengers associated with using single-dose second-generation anticoagulants against field rodents. In: Cowan DP, Freare CJ (eds) Advances in vertebrate pest management. Filander Verlag, Furth, pp 387–404
Naim M, Hafidzi MN, Azhar K, Jalila A (2010) Growth performance of nestling barn owls, Tyto Alba javanica in rat baiting area in Malaysia. ARPN J Agric Biol Sci 5:1–13
Newton I, Wyllie I, Freestone P (1990) Rodenticides in British barn owls. Environ Pollut 68:01–17
Newton I, Shore RF, Wyllie I, Birks JDS, Dale L (1999) Empirical evidence of side-effects of rodenticides on some predatory birds and mammals. In: Cowan DP, Feare CJ et al (eds) Advances in vertebrate pest management. Filander Verlag, Fürth, pp 347–367
Ntampakis D, Carter I (2005) Red Kite and rodenticides—a feeding experiment. Br Birds 98:411–416
Parmar G, Bratt H, Moore R, Batten PL (1987) Evidence for a common binding site in vivo for the retention of anticoagulants in rat liver. Hum Toxicol 6:431–432
Rammel CG, Hoogenboom JJL, Cotter M, Williams JM, Bell J (1984) Brodifacoum residues in target and non-target animals following rabbit poisoning trials. N Z J Exp Agric 12:107–111
Rattner BA, Horak KE, Warner SE, Day DD, Meteyer CU, Volker SF, Eisemann JD, Johnston JJ (2011) Acute toxicity, histopathology, and coagulopathy in American kestrels (Falco sparverius) following administration of the rodenticide diphacinone. Environ Toxicol Chem 30:1213–1222
Record CR, Marsh RE (1988) Rodenticide residues in animal carcasses and their relevance to secondary hazards. In: Proceedings of the thirteenth vertebrate pest conference, University of Nebraska, Lincoln, pp 163–168
Redfern R, Gill JE, Hadler MR (1976) Laboratory evaluation of WBA 8119 as a rodenticide for use against warfarin resistant and non-resistant rats and mice. J Hyg 77:419–426
Riley SPD, Bromley C, Poppenga RH, Uzal FA, Whited L, Sauvajot RM (2007) Anticoagulant exposure and notoedric mange in bobcats and mountain lions in urban southern California. J Wildlife Manage 71:1874–1884
Sánchez-Barbudo IS, Camarero PR, Mateo R (2012) Primary and secondary poisoning by anticoagulant rodenticides of non-target animals in Spain. Sci Total Environ 420:280–288
Sharp EA, Melton LM, Taylor MJ, Watson JE (2012) Pesticide poisoning of animals in 2011: A report of investigations in Scotland. Scottish Government Agriculture Food and Rural Communities Directorate, Edinburgh
Shore RF, Birks JDS, Afsar A, Wienburg CL, Kitchener AC (2003) Spatial and temporal analysis of second-generation anticoagulant rodenticide residues in polecats (Mustela putorius) from throughout their range in Britain, 1992–1999. Environ Pollut 122:183–193
Shore RF, Malcolm HM, McLennan D, Turk A, Walker LA, Wienburg CL, Burn AJ (2006) Did foot-and-mouth disease-control operations affect rodenticide exposure in raptors? J Wildl Manag 70:588–593
Shore RF, Walker LA, Thomas GO, Barber JL, Martin FR, Jones KC, Beresford NA, Rowland P, Pickup RW (2007) Review of the predatory bird monitoring scheme (PBMS) 2006. JNCC Report, No. 400
Spurr EB, Drew KW (1999) Invertebrates feeding on baits used for vertebrate pest control in New Zealand. N Z J Ecol 23:167–173
Stone WB, Okonlewski JC, Stedelin JR (1999) Poisoning of wildlife with anticoagulant rodenticides in New York, 1998–2001. Bull Environ Contam Toxicol 70:34–40
Thomas PJ, Mineau P, Shore RF, Champoux L, Martin PA, Wilson LK, Fitzgerald G, Elliot JE (2011) Second generation anticoagulant rodenticides in predatory birds: probabilistic characterisation of toxic liver concentrations and implications for predatory bird populations in Canada. Environ Int 37:914–920
Tosh DG, Shore RF, Jess S, Withers A, Bearhop S, Montgomery WI, McDonald RA (2011) User behaviour, best practice and the risks of non-target exposure associated with anticoagulant rodenticide use. J Environ Manag 92:1503–1508
Tosh DG, McDonald RA, Bearhop S, Llewellyn NR, Montgomery WI, Shore RF (2012) Rodenticide exposure in wood mouse and house mouse populations on farms and potential secondary risk to predators. Ecotoxicology 21:1325–1332
Vandenbroucke V, Bousquet-Melou A, De Backer P, Croubels S (2008) Pharmacokinetics of eight anticoagulant rodenticides in mice after single oral administration. J Vet Pharmacol Ther 31:437–445
Walker LA, Shore RF, Turk A, Pereira MG, Best J (2008) The predatory bird monitoring scheme: identifying chemical risks to top predators in Britain. Ambio 37:466–471
Walker LA, Llewellyln NR, Pereira MG, Potter ED, Molenaar FM, Sainsbury AW, Shore RF (2010) Anticoagulant rodenticides in predatory birds 2007 & 2008: a predatory bird monitoring scheme (PBMS) report. Centre for Ecology and Hydrology, Lancaster
Wyllie I (1995) Potential secondary poisoning of barn owls by rodenticides. Pestic Outlook 6:19–25
Acknowledgments
The authors would like to thank those who have assisted in the coordination and collection of rodenticide use data (Chris Bierley, Chris Griffiths, John Kerr, Gillian Reay, Louis Thomas, Andrew Walker and Jeremy Snowden) and those who conducted residue analyses (Anna Giela and Jennifer Watson). The authors would also like to thank the paper’s anonymous reviewers whose comments greatly improved the manuscript.
Conflict of interest
The post-registration surveillance of wildlife poisoning is funded by the pesticide industry under the Food and and Environmental Protection Act 1985 (FEPA) and the Control of Pesticides Regulations (COPR). The monitoring of rodenticide use is funded by the Scottish Government. The authors declare that they have no conflict of interest.
Ethical standards
All experiments comply with the current laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hughes, J., Sharp, E., Taylor, M.J. et al. Monitoring agricultural rodenticide use and secondary exposure of raptors in Scotland. Ecotoxicology 22, 974–984 (2013). https://doi.org/10.1007/s10646-013-1074-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1074-9