Abstract
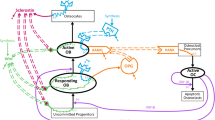
Osteoporosis is a metabolic bone disease resulting from increased bone resorption and characterized by low bone mass that leads to increased bone fragility and risk of fracture, particularly of the hip, spine and wrist. Bone resorption is dependent on receptor activator of NF-kappa B ligand (RANKL), which binds to RANK receptor on preosteoclasts to initiate osteoclastogenesis and maintains osteoclast function and survival. To neutralize the effects of RANKL, the body naturally produces the protein osteoprotegerin (OPG), which acts as a decoy receptor for RANKL and contributes to bone homeostasis. We describe the piecewise development of a three-compartment pharmacokinetic model with both linear and Michaelis–Menten eliminations, and an indirect pharmacodynamic response model to describe the pharmacokinetics and pharmacodynamics, respectively, of the fusion protein, Fc-osteoprotegerin (Fc-OPG), in healthy postmenopausal women. Subsequently, model verification was performed and used to address study design questions via simulation. The model was developed using data from eight cohorts (n = 13 subjects/cohort; Fc-OPG:placebo = 10:3) classified by dose level (0.1, 0.3, 1.0, or 3.0 mg/kg) and route of administration (intravenous [IV] or subcutaneous [SC]). Fc-OPG serum concentrations and urinary N-telopeptide/creatinine ratios (NTX) following both IV and SC administration were available. The model provided an adequate fit to the observed data and physiologically plausible parameter estimates. Model robustness was tested via a posterior predictive check with the model performing well in most cases. Subsequent clinical trial simulations demonstrated that a single 3.0-mg/kg SC dose of Fc-OPG would be expected to produce, at 14 days post-dose, a median NTX percentage change from baseline of −45% (with a 95% prediction interval ranging from −34% to −60%). Lastly, model ruggedness was evaluated using local and global sensitivity analysis methods. In conclusion, the model selection and simulation strategies we applied were rigorous, useful, and easily generalizable.
Similar content being viewed by others
Abbreviations
- DV:
-
Dependent variable
- FO:
-
First-order
- FOCE:
-
First-order conditional estimation
- IPRED:
-
Individual model prediction
- MOFV:
-
Minimum objective function value
- PRED:
-
Population model prediction
- WRES:
-
Weighted residuals
- OPG:
-
Osteoprotegerin
- RANKL:
-
Receptor activator of NF-kappa B ligand
- Fc-OPG:
-
Fc fused to OPG molecule
- NTX:
-
Urinary N-telopeptide/creatinine ratio
References
Akesson K (2003) New approaches to pharmacological treatment of osteoporosis. Bull World Health Organ 81: 657–664
Ettinger MP (2003) Aging bone and osteoporosis: strategies for preventing fractures in the elderly. Arch Intern Med 163: 2237–2246. doi:10.1001/archinte.163.18.2237
Sitruk-Ware R (2003) Alternatives for optimal hormone replacement therapy. Climacteric 6(Suppl 2): 11–16
Draper MW (2003) The role of selective estrogen receptor modulators (SERMs) in postmenopausal health. Ann N Y Acad Sci 997: 373–377. doi:10.1196/annals.1290.040
Rubin MR, Bilezikian JP (2003) The anabolic effects of parathyroid hormone therapy. Clin Geriatr Med 19: 415–432. doi:10.1016/S0749-0690(02)00074-5
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337–342. doi:10.1038/nature01658
Hofbauer LC, Kuhne CA, Viereck V (2004) The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact 4: 268–275
Bekker PJ, Holloway D, Nakanishi A, Arrighi M, Leese PT, Dunstan CR (2001) The effect of a single dose of osteoprotegerin in postmenopausal women. J Bone Miner Res 16: 348–360. doi:10.1359/jbmr.2001.16.2.348
Colburn WA (2003) Biomarkers in drug discovery and development: from target identification through drug marketing. J Clin Pharmacol 43: 329–341. doi:10.1177/0091270003252480
Gobburu JV, Sekar VJ (2002) Application of modeling and simulation to integrate clinical pharmacology knowledge across a new drug application. Int J Clin Pharmacol Ther 40: 281–288
Meibohm B, Derendorf H (2002) Pharmacokinetic/pharmacodynamic studies in drug product development. J Pharm Sci 91: 18–31. doi:10.1002/jps.1167
Holford NH, Kimko HC, Monteleone JP, Peck CC (2000) Simulation of clinical trials. Annu Rev Pharmacol Toxicol 40: 209–234. doi:10.1146/annurev.pharmtox.40.1.209
Blesch KS, Gieschke R, Tsukamoto Y, Reigner BG, Burger HU, Steimer JL (2003) Clinical pharmacokinetic/pharmacodynamic and physiologically based pharmacokinetic modeling in new drug development: the capecitabine experience. Invest New Drugs 21: 195–223. doi:10.1023/A:1023525513696
Gomeni R, Dangeli C, Bye A (2002) In silico prediction of optimal in vivo delivery properties using convolution-based model and clinical trial simulation. Pharm Res 19: 99–103. doi:10.1023/A:1013667718695
Nestorov I, Graham G, Duffull S, Aarons L, Fuseau E, Coates P (2001) Modeling and stimulation for clinical trial design involving a categorical response: a phase II case study with naratriptan. Pharm Res 18: 1210–1219. doi:10.1023/A:1010943430471
Research, USDoHaH, Administration, FaD, (CDER), CfDEaR & (CBER), CfBEaR guidance for industry population pharmacokinetics (1999)
Pillai G, Gieschke R, Goggin T, Jacqmin P, Schimmer RC, Steimer JL (2004) A semimechanistic and mechanistic population PK–PD model for biomarker response to ibandronate, a new bisphosphonate for the treatment of osteoporosis. Br J Clin Pharmacol 58: 618–631. doi:10.1111/j.1365-2125.2004.02224.x
Cremers S, Sparidans R, den HJ, Hamdy N, Vermeij P, Papapoulos S (2002) A pharmacokinetic and pharmacodynamic model for intravenous bisphosphonate (pamidronate) in osteoporosis. Eur J Clin Pharmacol 57: 883–890. doi:10.1007/s00228-001-0411-8
Beal SL, Sheiner LB (1992) NONMEM User Guide. In: NONMEM Project Group. University of California San Francisco, San Francisco
Steimer JL, Mallet A, Golmard JL, Boisvieux JF (1984) Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev 15: 265–292. doi:10.3109/03602538409015066
Carroll RJ, Ruppert D (1988) Transformation and weighting in regression. CRC Press
Jonsson EN, Karlsson MO (1999) Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58: 51–64. doi:10.1016/S0169-2607(98)00067-4
Yano Y, Beal SL, Sheiner LB (2001) Evaluating pharmacokinetic/pharmacodynamic models using the posterior predictive check. J Pharmacokinet Pharmacodyn 28: 171–192. doi:10.1023/A:1011555016423
Leamer EE (1990) Sensitivity analysis would help. In: Granger CWJ (eds) Modelling Economic Series. Clarendon Press, Oxford
Nestorov IA (1999) Sensitivity analysis of pharmacokinetic and pharmacodynamic systems: I. A structural approach to sensitivity analysis of physiologically based pharmacokinetic models. J Pharmacokinet Biopharm 27: 577–596. doi:10.1023/A:1020926525495
Piotrovskij V, Van Peer A (1997) A model with separate hepato-portal compartment (“first-pass” model): fitting to plasma concentration-time profiles in humans. Pharm Res 14: 230–237. doi:10.1023/A:1012065130597
Eastell R, Barton I, Hannon RA, Chines A, Garnero P, Delmas PD (2003) Relationship of early changes in bone resorption to the reduction in fracture risk with risedronate. J Bone Miner Res 18: 1051–1056. doi:10.1359/jbmr.2003.18.6.1051
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zierhut, M.L., Gastonguay, M.R., Martin, S.W. et al. Population PK–PD model for Fc-osteoprotegerin in healthy postmenopausal women. J Pharmacokinet Pharmacodyn 35, 379–399 (2008). https://doi.org/10.1007/s10928-008-9093-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-008-9093-5