Abstract
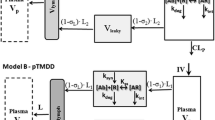
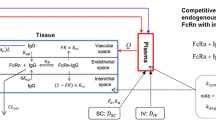
Minimal physiologically based pharmacokinetic (mPBPK) models provide a simple and sensible approach that incorporates physiological elements into pharmacokinetic (PK) analysis when only plasma data are available. With this modeling concept, a second-generation mPBPK model was further developed with specific accommodations for the unique PK properties of monoclonal antibodies (mAb). This study applied this model to extensively survey mAb PK in man in order to seek general perspectives on mAb distributional and elimination features. Profiles for 72 antibodies were successfully analyzed with this model. The model results provide assessment regarding: (1) predominant clearance site, in plasma or interstitial fluid (ISF); (2) mAb ISF concentrations in two groups of lumped tissues with continuous (V tight ) or fenestrated (V leaky ) vascular endothelium; (3) Transcapillary escape rate (TER), an indicator of systemic vascular permeability. For 93 % of surveyed mAbs, the model assuming clearance from plasma (CL p ) produced better or at least equivalent model performance than the model with clearance from ISF and yielded most consistent values of vascular reflection coefficients (σ1 and σ2) among all antibodies. The average mAb ISF concentration in V tight and V leaky at equilibrium was predicted to be about 6.8 and 37.9 % of that in plasma. A positive correlation was detected between plasma clearance and TER among most mAbs, which could be interpreted as both parameters having common determinants related to ISF tissue distribution in this model context. The mAbs with relative higher plasma clearance (>0.035 L/h/70 kg) did not reveal such positive correlation between clearance and TER, implying that the factors contributing to high clearance may not necessarily increase tissue distribution and penetration. In conclusion, this mPBPK model offers a more mechanistic approach for analyzing plasma mAb PK than compartment models and generates parameters providing useful intrinsic distributional and elimination insights for a large number of mAbs that were examined in man.







Similar content being viewed by others
References
Reichert JM (2012) Marketed therapeutic antibodies compendium. MAbs 4:413–415
Sliwkowski MX, Mellman I (2013) Antibody therapeutics in cancer. Science 341:1192–1198
Chan AC, Carter PJ (2010) Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol 10:301–316
Lin JH, Lu AY (1997) Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol Rev 49:403–449
Wang W, Wang EQ, Balthasar JP (2008) Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 84:548–558
Richter WF, Grimm HP, Theil FP (2011) Potential errors in the volume of distribution estimation of therapeutic proteins composed of differently cleared components. J Pharmacokinet Pharmacodyn 38:581–593
Garzone PD, Atkinson AJ Jr (2012) In search of physiologically based distribution volume estimates for macromolecules. Clin Pharmacol Ther 92:419–421
Cao Y, Jusko WJ (2012) Applications of minimal physiologically-based pharmacokinetic models. J Pharmacokinet Pharmacodyn 39:711–723
Cao Y, Balthasar JP, Jusko WJ (2013) Second-generation minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn 40:597–607
Sarin H (2010) Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J Angiogenes Res 2:14
Garg A, Balthasar JP (2007) Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn 34:687–709
Shah DK, Betts AM (2012) Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn 39:67–86
Wagshul ME, Johnston M (2013) The brain and the lymphatic system. Immunology of the Lymphatic System. Springer, New York, pp 143–164
Davies B, Morris T (1993) Physiological parameters in laboratory animals and humans. Pharm Res 10:1093–1095
Stucker O, Pons-Himbert C, Laemmel E (2008) Towards a better understanding of lymph circulation. Phlebolymphology. 15:31–36
Wiig H, Tenstad O (2001) Interstitial exclusion of positively and negatively charged IgG in rat skin and muscle. Am J Physiol Heart Circ Physiol 280:H1505–H1512
Wiig H, Kaysen GA, al-Bander HA, De Carlo M, Sibley L, Renkin EM (1994) Interstitial exclusion of IgG in rat tissues estimated by continuous infusion. Am J Physiol 266:H212–H219
D’Argenio DZ, Schumitzky A (2009) ADAPT V User’s Guide: Pharmacokinetic/Pharmacodynamic System Analysis Software. Biomedical Simulations Resource, Los Angeles
Boswell CA, Tesar DB, Mukhyala K, Theil FP, Fielder PJ, Khawli LA (2010) Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconjug Chem 21:2153–2163
Davies PF, Ross R (1978) Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol 79:663–671
Bumbaca D, Boswell CA, Fielder PJ, Khawli LA (2012) Physiochemical and biochemical factors influencing the pharmacokinetics of antibody therapeutics. AAPS J 14:554–558
Schmidt MM, Wittrup KD (2009) A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther 8:2861–2871
Golay J, Semenzato G, Rambaldi A, Foa R, Gaidano G, Gamba E, Pane F, Pinto A, Specchia G, Zaja F, Regazzi M (2013) Lessons for the clinic from rituximab pharmacokinetics and pharmacodynamics. MAbs 5:826–837
Tan AR, Moore DF, Hidalgo M, Doroshow JH, Poplin EA, Goodin S, Mauro D, Rubin EH (2006) Pharmacokinetics of cetuximab after administration of escalating single dosing and weekly fixed dosing in patients with solid tumors. Clin Cancer Res 12:6517–6522
Cao YG and Jusko WJ (2014) Incorporating target-mediated drug disposition in a minimal physiologically-based pharmacokinetic model for monoclonal antibodies. J Pharmacokinet Pharmacodyn. doi:10.1007/s10928-014-9372-2
Acknowledgments
This research was supported by the National Institutes of Health Grant GM57980 and the UB Center for Protein Therapeutics.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cao, Y., Jusko, W.J. Survey of monoclonal antibody disposition in man utilizing a minimal physiologically-based pharmacokinetic model. J Pharmacokinet Pharmacodyn 41, 571–580 (2014). https://doi.org/10.1007/s10928-014-9374-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10928-014-9374-0