No Heading
Purpose.
The human proton-coupled small peptide carrier (hPEPT1) is a low-affinity, high-capacity transporter with broad substrate specificity. We have taken an iterative in vitro and in silico approach to the discovery of molecules with hPEPT1 affinity.
Methods.
A pharmacophore-based approach was taken to identifying hPEPT1 inhibitors. The well-characterized and relatively high affinity ligands Gly-Sar, bestatin, and enalapril were used to generate a common features (HIPHOP) pharmacophore. This consisted of two hydrophobic features, a hydrogen bond donor, acceptor, and a negative ionizable feature.
Results.
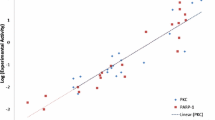
The pharmacophore was used to search the Comprehensive Medicinal Chemistry (CMC) database of more than 8000 drug-like molecules and retrieved 145 virtual hits mapping to the pharmacophore features. The highest scoring compounds within this set were selected and tested in a stably transfected CHO-hPepT1 cell model. The antidiabetic repaglinide and HMG CoA reductase inhibitor fluvastatin were found to inhibit hPEPT1 with sub-millimolar potency (IC50 178 ± 1.0 and 337 ± 4 μM, respectively). The pharmacophore was also able to identify known hPEPT1 substrates and inhibitors in further database mining of more than 500 commonly prescribed drugs.
Conclusions.
This study demonstrates the potential of combining computational and in vitro approaches to determine the affinity of compounds for hPEPT1 and, in turn, provides insights into key molecular interactions with this transporter.
Similar content being viewed by others
References
1. C. W. Andrews, L. Bennett, and L. X. Yu. Predicting human oral bioavailability of a compound: development of a novel quantitative structure-bioavailability relationship. Pharm. Res. 17:639–644 (2000).
2. D. F. Veber, S. R. Johnson, H.-Y. Cheng, B. R. Smith, K. W. Ward, and K. D. Kopple. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45:2615–2623 (2002).
3. E. Y. Zhang, M. A. Phelps, C. Cheng, S. Ekins, and P. W. Swaan. Modeling of active transport systems. Adv. Drug Del. Rev. 54:329–354 (2002).
4. S. Ekins and P. W. Swaan. Computational models for enzymes, transporters, channels and receptors relevant to absorption, distribution, metabolism, excretion and toxicology. Rev. Comp. Chem. 20:333–415 (2004).
5. S. Gebauer, I. Knutter, B. Hartrodt, M. Brandsch, K. Neubert, and I. Thondorf. Three-dimensional quantitative structure-activity relationship analyses of peptide substrates of the mammalian H+/peptide cotransporter PEPT1. J. Med. Chem. 46:5725–5734 (2003).
6. N. J. Snyder, L. B. Tabas, D. M. Berry, D. C. Duckworth, D. O. Spry, and A. H. Dantzig. Structure-activity relationship of carbacephalosporins and cephalosporins: antibacterial activity and interaction with the intestinal proton-dependent dipeptide transport carrier of Caco-2 cells. Antimicrob. Agents Chemother. 41:1649–1657 (1997).
7. F. H. Leibach and V. Ganapathy. Peptide transporters in the intestine and the kidney. Annu. Rev. Nutr. 16:99–119 (1996).
8. H. K. Han, J. K. Rhie, D. M. Oh, G. Saito, C. P. Hsu, B. H. Stewart, and G. L. Amidon. CHO/hPEPT1 cells overexpressing the human peptide transporter (hPEPT1) as an alternative in vitro model for peptidomimetic drugs. J. Pharm. Sci. 88:347–350 (1999).
9. E. Y. Zhang, D. J. Fu, Y. A. Pak, T. Stewart, N. Mukhopadhyay, S. A. Wrighton, and K. M. Hillgren. Genetic polymorphisms in human proton-dependent dipeptide transporter PEPT1: implications for the functional role of Pro586. J. Pharmacol. Exp. Ther. 310:437–445 (2004).
10. M. Brandsch, I. Knutter, and F. H. Leibach. The intestinal H+/peptide symporter PEPT1: structure-affinity relationships. Eur. J. Pharm. Sci. 21:53–60 (2004).
11. O. O. Clement and A. T. Mehl. HipHop: Pharmacophore based on multiple common-feature alignments. In O. F. Guner (ed.), Pharmacophore Perception, Development, and Use in Drug Design, IUL, San Diego, CA, 2000, pp. 69–84.
12. C. Y. Yang, A. H. Dantzig, and C. Pidgeon. Intestinal peptide transport systems and oral drug availability. Pharm. Res. 16:1331–1343 (1999).
13. L. G. Gomella and S. A. Haist. Clinician’s Pocket Drug Reference, McGraw-Hill, New York, 2004.
14. K. M. Covitz, G. L. Amidon, and W. Sadee. Human dipeptide transporter, hPEPT1, stably transfected into Chinese hamster ovary cells. Pharm. Res. 13:1631–1634 (1996).
15. P. W. Swaan, B. C. Koops, E. E. Moret, and J. J. Tukker. Mapping the binding site of the small intestinal peptide carrier (PepT1) using comparative molecular field analysis. Receptors Channels 6:189–200 (1998).
16. P. W. Swaan, F. C. Szoka Jr., and S. Oie. Molecular modeling of the intestinal bile acid carrier: a comparative molecular field analysis study. J. Comput. Aided Mol. Des. 11:581–588 (1997).
17. J. Li and I. J. Hidalgo. Molecular modeling study of structural requirements for the oligopeptide transporter. J. Drug Target. 4:9–17 (1996).
18. F. Doring, J. Will, S. Amasheh, W. Clauss, H. Ahlbrecht, and H. Daniel. Minimal molecular determinants of substrates for recognition by the intestinal peptide transporter. J. Biol. Chem. 273:23211–23218 (1998).
19. M. E. Ganapathy, W. Huang, H. Wang, V. Ganapathy, and F. H. Leibach. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem. Biophys. Res. Commun. 246:470–475 (1998).
20. P. V. Balimane, I. Tamai, A. Guo, T. Nakanishi, H. Kitada, F. H. Leibach, A. Tsuji, and P. J. Sinko. Direct evidence for peptide transporter (PepT1)-mediated uptake of a nonpeptide prodrug, valacyclovir. Biochem. Biophys. Res. Commun. 250:246–251 (1998).
21. P. D. Bailey, C. A. R. Boyd, J. R. Bronk, I. D. Collier, D. Meredith, K. M. Morgan, and C. S. Temple. How to make drugs orally active: a substrate template for peptide transporter PepT1. Angew. Chem. Int. Ed. Engl. 39:506–508 (2000).
22. B. Bretschneider, M. Brandsch, and R. Neubert. Intestinal transport of β-lactam antibiotics: analysis of the affinity at the H+/peptide symporter (PEPT1), the uptake into caco-2 cell monolayers and the transepithelial flux. Pharm. Res. 16:55–61 (1999).
23. V. A. Moore, W. J. Irwin, P. Timmins, P. A. Lambert, S. Chong, S. A. Dando, and R. A. Morrison. A rapid screening system to determine drug affinities for the intestinal dipeptide transporter 2: Affinities of ACE inhibitors. Int. J. Pharm. 210:29–44 (2000).
24. F. L. Tse, J. M. Jaffe, and A. Troendle. Pharmacokinetics of fluvastatin after single and multiple doses in normal volunteers. J. Clin. Pharmacol. 32:630–638 (1992).
25. W. E. Lipton, Y. N. Li, M. K. Younoszai, and L. D. Stegink. Intestinal absorption of aspartame decomposition products in adult rats. Metabolism 40:1337–1345 (1991).
26. S. Itagaki, Y. Saito, S. Kubo, Y. Otsuka, Y. Yamamoto, M. Kobayashi, T. Hirano, and K. Iseki. H+-dependent transport mechanism of nateglinide in the brush-border membrane of the rat intestine. J. Pharmacol. Exp. Ther. 312(1):77–82 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ekins, S., Johnston, J., Bahadduri, P. et al. In Vitro and Pharmacophore-Based Discovery of Novel hPEPT1 Inhibitors. Pharm Res 22, 512–517 (2005). https://doi.org/10.1007/s11095-005-2505-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2505-y