Abstract
Abstract
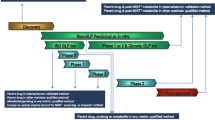
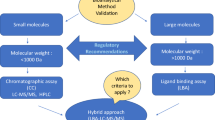
The Third AAPS/FDA Bioanalytical Workshop, entitled “Quantitative Bioanalytical Methods Validation and Implementation: Best Practices for Chromatographic and Ligand Binding Assays” was held on May 1–3, 2006 in Arlington, VA. The format of this workshop consisted of presentations on bioanalytical topics, followed by discussion sessions where these topics could be debated, with the goal of reaching consensus, or identifying subjects where addition input or clarification was required. The discussion also addressed bioanalytical validation requirements of regulatory agencies, with the purpose of clarifying expectations for regulatory submissions. The proceedings from each day were reviewed and summarized in the evening sessions among the speakers and moderators of the day. The consensus summary was presented back to the workshop on the last day and was further debated. This communication represents the distillate of the workshop proceedings and provides the summary of consensus reached and also contains the validation topics where no consensus was reached.
Similar content being viewed by others
References
V. P. Shah, K. K. Midha, and S. V. Dighe. Analytical methods validation: bioavailability, bioequivalence and pharmacokinetic studies (conference report). Eur. J. Drug Metab Pharmacokinet. 16(4):249–255 (1992).
V. P. Shah, K. K. Midha, J. W. A. Findlay, H. M. Hill, J. D. Hulse, I. J. McGilveray, G. McKay, K. J. Miller, R. N. Patnaik, M. L. Powell, A. Tonelli, C. T. Viswanathan, and A. Yacobi. Bioanalytical method validation—a revisit with a decade of progress. Pharm. Res. 17(12):1551–1557 (2000).
Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation, US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Rockville, MD, 2001.
B. DeSilva, W. Smith, R. Weiner, M. Kelley, J. Smolec, B. Lee, M. Khan, R. Tacey, H. Hill, and A. Celniker. Recommendations for the bioanalytical method validation of ligand-binding assays to support pharmacokinetic assessments of macromolecules. Pharm. Res. 20(11):1885–1900 (2003).
Food and Drug Administration. Draft Guidance for Industry: Safety Testing of Drug Metabolites, US Department of Health and Human Services, FDA, Center for Drug Evaluation and Research, Rockville, MD, 2005.
Acknowledgement
Contributors to this report include: Mark Arnold (Bristol-Myers Squibb Inc., Princeton, NJ 08543), Bruno Boulanger (Eli Lilly, Mont-Saint-Guibert, Belgium), Ronald Bowsher (LINCO Diagnostic Services, Saint Charles, MO 63304), Ajai Chaudhary (Lilly Research Laboratories, Indianapolis, IN 46285), Daksha Desai-Krieger (Johnson & Johnson Inc., Spring House, PA 19477), Binodh DeSilva (Amgen Inc., Thousand Oaks, CA 91320), John Findlay (Pfizer Global Research and Development, Groton, CT 06340), Boris Gorovits (Wyeth, Pearl River, NY 10965), Howard M. Hill (Huntingdon Life Sciences, Huntingdon, Cambridge, PE17 5HS, UK), Thomas Huggins (Procter & Gamble Pharmaceuticals, Mason, OH 45040), Christopher James (Amgen Inc., Thousand Oaks, CA 91320), John Kamerud (Lilly Research Laboratories, Indianapolis, IN 46285), Marian Kelley (Centocor Inc., Wayne, PA 19087), Masood Khan (Covance Laboratories, Chantilly, VA 20151), Jean Lee (Amgen Inc., Thousand Oaks, CA 91320), Zhenmin Liang (Hoffmann-La Roche Inc., Nutley, NJ 07110), Steve Lowes (Advion BioSciences Inc., Ithaca, NY 14850), Kamal Midha (University of Saskatchewan, Saskatoon, SK S7N-3R2 CANADA), Eric Ormsby (Health Canada, Ottawa, Ontario, CANADA), Marie Rock (Midwest BioResearch LLC, Oak Park, IL 60302), Bonita Rup (Wyeth Research, Andover, MA 01810), Michael Skelly (Food and Drug Administration, Center for Drug Evaluation and Research, Rockville, MD 20857; see article footnote), Wendell Smith (Bowsher Brunelle and Smith, LLC, IN 46140), Jo Marie Smolec (Alta Analytical Laboratories Inc., San Diego, CA 92124), Richard Tacey (PPD Development, Richmond, VA 23233), Steve Unger (Bristol-Myers Squibb, New Brunswick, NJ 08903), Patrick Vallano (Mylan Pharmaceuticals Inc., Morgantown, WV 26505), Eric Woolf (Merck Research Labs, West Point, PA 19486), Jing-Tao Wu (Millennium Pharmaceuticals Inc., Cambridge, MA 02139), Martin Yau (Food and Drug Administration, Center for Drug Evaluation and Research, Rockville, MD 20857; see article footnote).
Author information
Authors and Affiliations
Corresponding author
Additional information
The views expressed in this article are those of the authors and do not reflect official policy at the FDA. No official endorsement by the FDA is intended or should be inferred.
Rights and permissions
About this article
Cite this article
Viswanathan, C.T., Bansal, S., Booth, B. et al. Quantitative Bioanalytical Methods Validation and Implementation: Best Practices for Chromatographic and Ligand Binding Assays. Pharm Res 24, 1962–1973 (2007). https://doi.org/10.1007/s11095-007-9291-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9291-7