Abstract
Purpose
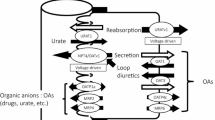
To compare the interaction of human organic anion transporter hOAT4 with PDZ proteins between kidney cells and placental cells.
Materials and Methods
PDZ proteins PDZK1 and NHERF1 were transfected into kidney LLC-PK1 cells and placental BeWo cells expressing hOAT4 or hOAT4-Δ, which lacks the PDZ consensus binding site. The interaction of PDZK1 and NHERF1 with hOAT4 and hOAT4-Δ was investigated by measurement of [3H] estrone sulfate uptake, cell surface and total cell expression of hOAT4.
Results
PDZK1 and NHERF1 enhanced hOAT4 activity in LLC-PK1 cells by increasing the cell surface expression of the transporter. In contrasts, these two PDZ proteins had no effect on hOAT4 activity in BeWo cells.
Conclusion
The interaction of PDZ proteins with hOAT4 may be cell-specific. In placenta, a different set of interacting proteins from PDZK1 and NHERF1 may be required to modulate hOAT4 activity.





Similar content being viewed by others
References
B. C. Burckhardt, and G. Burckhardt. Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev. Physiol. Biochem. Pharmacol. 146:95–158 (2003).
S. A. Eraly, K. T. Bush, R. V. Sampogna, V. Bhatnagar, and S. K. Nigam. The molecular pharmacology of organic anion transporters: from DNA to FDA? Mol. Pharmacol. 65:479–487 (2004).
T. Sekine, H. Miyazaki, and H. Endou. Molecular physiology of renal organic anion transporters. Am. J. Physiol. Renal. Physiol. 290:F251–261 (2006).
D. H. Sweet. Organic anion transporter (Slc22a) family members as mediators of toxicity. Toxicol. Appl. Pharmacol. 204:198–215 (2005).
G. You. Structure, function, and regulation of renal organic anion transporters. Med. Res. Rev. 22:602–616 (2002).
S. H. Cha, T. Sekine, H. Kusuhara, E. Yu, J. Y. Kim, D. K. Kim, Y. Sugiyama, Y. Kanai, and H. Endu. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J. Biol. Chem. 275:4507–4512 (2000).
S. Ekaratanawong, N. Anzai, P. Jutabha, H. Miyazaki, R. Noshiro, M. Takeda, Y. Kanai, S. Sophasan, and H. Endou. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J. Pharmacol. Sci. 94:297–304 (2004).
B. Ugele, M. V. St-Pierre, M. Pihusch, A. Bahn, and P. Hantschmann. Characterization and identification of steroid sulfate transporters of human placenta. Am. J. Physiol. Endocrinol. Metab. 284:E390–398 (2003).
T. Rabe, R. Hosch, and B. Runnebaum. Diagnosis of intrauterine fetal growth retardation (IUGR) and placental insufficiency by a dehydroepiandrosterone sulfate (DHAS) loading test. Biol. Res. Pregnancy. Perinatol. 4:130–136 (1983).
F. Zhou, W. Xu, M. Hong, Z. Pan, P. J. Sinko, J. Ma, and G. You. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol. Pharmacol. 67:868–876 (2005).
H. Miyazaki, N. Anzai, S. Ekaratanawong, T. Sakata, H. J. Shin, P. Jutabha, T. Hirata, X. He, H. Nonoguchi, K. Tomita, Y. Kanai, and H. Endou. Modulation of renal apical organic anion transporter 4 function by two PDZ domain-containing proteins. J. Am. Soc. Nephrol. 16(12):3498–506 (2005).
N. Anzai, H. Miyazaki, R. Noshiro, S. Khamdang, A. Chairoungdua, H. J. Shin, A. Enomoto, S. Sakamoto, T. Hirata, K. Tomita, Y. Kanai, and H. Endou. The multivalent PDZ domain-containing protein PDZK1 regulates transport activity of renal urate-anion exchanger URAT1 via its C terminus. J. Biol. Chem. 279(44):45942–45950 (2004).
P. Wang, J. J. Wang, Y. Xiao, J. W. Murray, P. M. Novikoff, R. H. Angeletti, G. A. Orr, D. Lan, D. L. Silver, and A. W. Wolkoff. Interaction with PDZK1 is required for expression of organic anion transporting protein 1A1 on the hepatocyte surface. J. Biol Chem. 280(34):30143–30149 (2005).
F. Zhou, M. Hong, G. You. Regulation of human organic anion transporter 4 (hOAT4) by progesterone and protein kinase C in human placental BeWo cells. Am. J. Physiol. Endocrinol. Metab. (2007) (in press).
Acknowledgment
This work was supported by Grant R01-DK 60034 from the National Institutes of Health (to Dr. Guofeng You).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, F., Xu, W., Tanaka, K. et al. Comparison of the Interaction of Human Organic Anion Transporter hOAT4 with PDZ Proteins between Kidney Cells and Placental Cells. Pharm Res 25, 475–480 (2008). https://doi.org/10.1007/s11095-007-9359-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9359-4