ABSTRACT
Purpose
To develop a pharmacokinetic-pharmacodynamic disease progression (PK/PD/DIS) model to characterize the effect of etanercept in collagen-induced arthritis (CIA) rats on rheumatoid arthritis (RA) progression.
Methods
The CIA rats received either 5 mg/kg intravenous (IV), 1 mg/kg IV, or 5 mg/kg subcutaneous (SC) etanercept at day 21 post-disease induction. Effect on disease progression was measured by paw swelling. Plasma concentrations of etanercept were assayed by enzyme-linked immunosorbent assay (ELISA). PK profiles were fitted first; parameter estimates were applied to fit paw edema data for PD and DIS-related parameter estimation using ADAPT 5 software.
Results
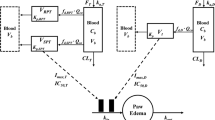
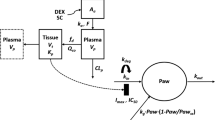
The model contained a two-compartment PK model with Michaelis-Menten elimination. For SC administration, two additional mathematical functions for absorption were added. The disease progression component was an indirect response model with a time-dependent change in paw edema production rate constant (k in ) assumed to be inhibited by etanercept.
Conclusions
Etanercept has modest effects on paw swelling in CIA rats. The PK and PD profiles were well described by the developed PK/PD/DIS model, which may be used for other anti-cytokine biologic agents for RA.




Similar content being viewed by others
REFERENCES
Rheumatoid Arthritis. http://www.cdc.gov/arthritis/basics/rheumatoid.htm.
Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117:244–79.
Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16.
Roy A, Mould DR, Wang XF, Tay L, Raymond R, Pfister M. Modeling and simulation of abatacept exposure and interleukin-6 response in support of recommended doses for rheumatoid arthritis. J Clin Pharmacol. 2007;47:1408–20.
Feely MG, Erickson A, O’Dell JR. Therapeutic options for rheumatoid arthritis. Expert Opin Pharmacother. 2009;10:2095–106.
Riley K. FDA approves new drug for rheumatoid arthritis. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm197108.htm.
Shingu M, Nagai Y, Isayama T, Naono T, Nobunaga M. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993;94:145–9.
Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–72.
Jarvis B, Faulds D. Etanercept: a review of its use in rheumatoid arthritis. Drugs. 1999;57:945–66.
Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2006;63:2451–65.
Enbrel (etanercept) solution for subcutaneous use. http://www.enbrel.com/documents/ENBREL-Prescribing-Information.pdf.
Zanella JM, Burright EN, Hildebrand K, Hobot C, Cox M, Christoferson L, et al. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine (Phila Pa 1976). 2008;33:227–34.
Earp JC, Dubois DC, Molano DS, Pyszczynski NA, Keller CE, Almon RR, et al. Modeling corticosteroid effects in a rat model of rheumatoid arthritis I: Mechanistic disease progression model for the time course of collagen-induced arthritis in Lewis rats. J Pharmacol Exp Ther. 2008;326:532–45.
Earp JC, Dubois DC, Molano DS, Pyszczynski NA, Almon RR, Jusko WJ. Modeling corticosteroid effects in a rat model of rheumatoid arthritis II: Mechanistic pharmacodynamic model for dexamethasone effects in Lewis rats with collagen-induced arthritis. J Pharmacol Exp Ther. 2008;326:546–54.
Earp JC, Dubois DC, Almon RR, Jusko WJ. Quantitative dynamic models of arthritis progression in the rat. Pharm Res. 2009;26:196–203.
Post TM, Freijer JI, DeJongh J, Danhof M. Disease system analysis: basic disease progression models in degenerative disease. Pharm Res. 2005;22:1038–49.
D’Argenio DZ, Schumitzky A, Wang X. ADAPT 5 user’s guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. 2009.
Ramakrishnan R, Cheung WK, Farrell F, Joffee L, Jusko WJ. Pharmacokinetic and pharmacodynamic modeling of recombinant human erythropoietin after intravenous and subcutaneous dose administration in cynomolgus monkeys. J Pharmacol Exp Ther. 2003;306:324–31.
Wiens A, Venson R, Correr CJ, Otuki MF, Pontarolo R. Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy 30:339–53.
Hsu YH, Chang MS. Interleukin-20 antibody is a potential therapeutic for experimental arthritis. Arthritis Rheum.
Nestorov I. Clinical pharmacokinetics of TNF antagonists: how do they differ? Semin Arthritis Rheum. 2005;34:12–8.
Zhou H. Clinical pharmacokinetics of etanercept: a fully humanized soluble recombinant tumor necrosis factor receptor fusion protein. J Clin Pharmacol. 2005;45:490–7.
Filler SG, Solis NV, Guo J, Doellgast G, Ruiz-Garcia A, Pan WJ. Pharmacokinetics of murine p75-Fc fusion protein and MP6-XT22 anti-murine TNF-alpha mAb in mice. J Investig Dermatol Symp Proc. 2007;12:52–6.
Korth-Bradley JM, Rubin AS, Hanna RK, Simcoe DK, Lebsack ME. The pharmacokinetics of etanercept in healthy volunteers. Ann Pharmacother. 2000;34:161–4.
Lee H, Kimko HC, Rogge M, Wang D, Nestorov I, Peck CC. Population pharmacokinetic and pharmacodynamic modeling of etanercept using logistic regression analysis. Clin Pharmacol Ther. 2003;73:348–65.
Anderson PJ. Tumor necrosis factor inhibitors: clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. 2005;34:19–22.
Clinical Pharmacokinetics Review of BLA 98–0286 (Enbrel, TNFR:Fc). http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/ucm088681.pdf.
Mager DE, Jusko WJ. Receptor-mediated pharmacokinetic/pharmacodynamic model of interferon-beta 1a in humans. Pharm Res. 2002;19:1537–43.
Mould DR, Green B. Pharmacokinetics and pharmacodynamics of monoclonal antibodies: concepts and lessons for drug development. BioDrugs. 24:23–39.
Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm. 1993;21:457–78.
Luross JA, Williams NA. The genetic and immunopathological processes underlying collagen-induced arthritis. Immunology. 2001;103:407–16.
Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2004;1024:138–46.
Soller JT, Murua-Escobar H, Willenbrock S, Janssen M, Eberle N, Bullerdiek J, et al. Comparison of the human and canine cytokines IL-1(alpha/beta) and TNF-alpha to orthologous other mammalians. J Hered. 2007;98:485–90.
Roord ST, Zonneveld-Huijssoon E, Le T, Yung GP, Koffeman E, Ronaghy A, et al. Modulation of T cell function by combination of epitope specific and low dose anticytokine therapy controls autoimmune arthritis. PLoS One. 2006;1:e87.
Byon W, Fletcher CV, Brundage RC. Impact of censoring data below an arbitrary quantification limit on structural model misspecification. J Pharmacokinet Pharmacodyn. 2008;35:101–16.
Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28:481–504.
Duval V, Karlsson MO. Impact of omission or replacement of data below the limit of quantification on parameter estimates in a two-compartment model. Pharm Res. 2002;19:1835–40.
ACKNOWLEDGMENTS
This work was supported by funding from the UB Center for Protein Therapeutics, NIH Grant GM 24211, a fellowship for Ms. Lon from Amgen, Inc., and fellowship support for Dr. Liu from Hoffman-LaRoche Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lon, HK., Liu, D., Zhang, Q. et al. Pharmacokinetic-Pharmacodynamic Disease Progression Model for Effect of Etanercept in Lewis Rats with Collagen-Induced Arthritis. Pharm Res 28, 1622–1630 (2011). https://doi.org/10.1007/s11095-011-0396-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0396-7