ABSTRACT
Purpose
Anti-Aβ Ab2 (Ab2) is a humanized monoclonal antibody against amino acids 3–6 of primate (but not rodent) amyloid β (Aβ) and is being evaluated for the treatment of Alzheimer’s disease (AD). This study was conducted to predict the human pharmacokinetics of Ab2.
Methods
In vivo PK profile of Ab2 in preclinical species and in vitro mechanistic studies in preclinical and human systems were used for pharmacokinetic predictions.
Results
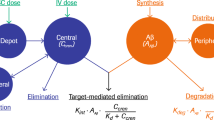
In Tg2576 and PSAPP mice that have ~100-fold higher circulating levels of human Aβ compared to humans, elimination of Ab2 was target-mediated, such that exposure was 5–10 fold lower compared to wild-type rodents or to PDAPP mice that have human Aβ concentrations in plasma similar to humans. In cynomolgus monkeys, the t1/2 of Ab2 was faster (<2.5 days) compared to that of the control antibody (~13 days). The fast elimination of Ab2 in cynomolgus monkeys was linked to off-target binding to cynomolgus monkey fibrinogen that was also causing incomplete recovery of Ab2 in cynomolgus monkey serum in blood partitioning experiments. Ab2 had significantly weaker to undetectable binding to human (and mouse) fibrinogen and had good recovery in human serum in blood partitioning experiments.
Conclusions
These data predict that elimination of Ab2 in healthy or AD humans is expected to be slow, with t1/2 similar to that observed for other humanized antibodies.



Similar content being viewed by others
REFERENCES
Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9.
Games D, Bard F, Grajeda H, Guido T, Khan K, Soriano F, et al. Prevention and reduction of AD-type pathology in PDAPP mice immunized with A beta 1–42. Ann N Y Acad Sci. 2000;920:274–84.
Lemere CA, Maier M, Jiang L, Peng Y, Seabrook TJ. Amyloid-beta immunotherapy for the prevention and treatment of Alzheimer disease: lessons from mice, monkeys, and humans. Rejuvenation Res. 2006;9:77–84.
Jicha GA. Is passive immunization for Alzheimer’s disease ‘alive and well’ or ‘dead and buried’? Expert Opin Biol Ther. 2009;9:481–91.
Wilcockand DM, Colton CA. Anti-amyloid-beta immunotherapy in Alzheimer’s disease: relevance of transgenic mouse studies to clinical trials. J Alzheimers Dis. 2008;15:555–69.
Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, et al. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4:97–100.
Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, et al. Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997;17:7053–9.
Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer’s disease. J Neurosci. 2001;21:372–81.
Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5161–6.
Golde TE, Das P, Levites Y. Quantitative and mechanistic studies of abeta immunotherapy. CNS Neurol Disord Drug Targets. 2009;8:31–49.
Levites Y, Smithson LA, Price RW, Dakin RS, Yuan B, Sierks MR, et al. Insights into the mechanisms of action of anti-Abeta antibodies in Alzheimer’s disease mouse models. Faseb J. 2006;20:2576–8.
Dodel RC, Hampel H, Du Y. Immunotherapy for Alzheimer’s disease. Lancet Neurol. 2003;2:215–20.
Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–93.
Gaugler MN, Tracy J, Kuhnle K, Crameri A, Nitsch RM, Mohajeri MH. Modulation of Alzheimer’s pathology by cerebro-ventricular grafting of hybridoma cells expressing antibodies against Abeta in vivo. FEBS Lett. 2005;579:753–6.
Karlnoski RA, Rosenthal A, Alamed J, Ronan V, Gordon MN, Gottschall PE, et al. Deglycosylated anti-Abeta antibody dose-response effects on pathology and memory in APP transgenic mice. J Neuroimmune Pharmacol. 2008;3:187–97.
Vugmeyster Y, DeFranco D, Szklut P, Wang Q, Xu X. Biodistribution of [125I]-labeled therapeutic proteins: application in protein drug development beyond oncology. J Pharm Sci. 99:1028–45.
Janus C, Westaway D. Transgenic mouse models of Alzheimer’s disease. Physiol Behav. 2001;73:873–86.
Lichtlen P, Mohajeri MH. Antibody-based approaches in Alzheimer’s research: safety, pharmacokinetics, metabolism, and analytical tools. J Neurochem. 2008;104:859–74.
Yu P, Oberto G. Alzheimer’s disease: transgenic mouse models and drug assessment. Pharmacol Res. 2000;42:107–14.
Davis CB, Garver EM, Kwok DC, Urbanski JJ. Disposition of metabolically radiolabeled CE9.1–a macaque-human chimeric anti-human CD4 monoclonal antibody–in transgenic mice bearing human CD4. Drug metabolism and disposition: the biological fate of chemicals. 1996;24:1032–7.
Putnam WS, Li J, Haggstrom J, Ng C, Kadkhodayan-Fischer S, Cheu M, et al. Use of quantitative pharmacology in the development of HAE1, a high-affinity anti-IgE monoclonal antibody. The AAPS Journal. 2008;10:425–30.
Ghiso J, Shayo M, Calero M, Ng D, Tomidokoro Y, Gandy S, et al. Systemic catabolism of Alzheimer’s Abeta40 and Abeta42. J Biol Chem. 2004;279:45897–908.
Amris A, Amris CJ. Turnover and distribution of 131-iodine-labelled human fibrinogen. Thromb Diath Haemorrh. 1964;11:404–22.
Areekul S, Devakul K, Chongsuphajaisiddhi T, Vivatanasesth P, Kanakakorn K, Kasemsuth R. Metabolism of 131 I-labelled fibrinogen in monkeys infected with Plasmodium coatneyi. Southeast Asian J Trop Med Public Health. 1971;2:455–61.
Moza AK, Sapru RP. Turnover of radio-iodinated and biosynthetically labelled fibrinogen in rhesus monkeys. Indian J Med Res. 1982;76:609–17.
Negrier C, Rothschild C, Goudemand J, Borg JY, Claeyssens S, Alessi MC, et al. Pharmacokinetics and pharmacodynamics of a new highly secured fibrinogen concentrate. J Thromb Haemost. 2008;6:1494–9.
Tytgat GN, Collen D, Verstraete M. Metabolism of fibrinogen in cirrhosis of the liver. J Clin Invest. 1971;50:1690–701.
Bonfanti U, Lamparelli D, Colombo P, Bernardi C. Hematology and serum chemistry parameters in juvenile cynomolgus monkeys (Macaca fascicularis) of Mauritius origin: comparison between purpose-bred and captured animals. J Med Primatol. 2009.
Stern RA, Trojanowski JQ, Lee VM. Antibodies to the beta-amyloid peptide cross-react with conformational epitopes in human fibrinogen subunits from peripheral blood. FEBS Lett. 1990;264:43–7.
Merkle DL, Cheng CH, Castellino FJ, Chibber BA. Modulation of fibrin assembly and polymerization by the beta-amyloid of Alzheimer’s disease. Blood Coagul Fibrinolysis. 1996;7:650–8.
Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron. 66:695–709.
Ghiso J, Rostagno A, Gardella JE, Liem L, Gorevic PD, Frangione B. A 109-amino-acid C-terminal fragment of Alzheimer’s-disease amyloid precursor protein contains a sequence, -RHDS-, that promotes cell adhesion. Biochem J. 1992;288(Pt 3):1053–9.
Springer TA, Zhu J, Xiao T. Structural basis for distinctive recognition of fibrinogen gamma C peptide by the platelet integrin alphaIIbbeta3. J Cell Biol. 2008;182:791–800.
ACKNOWLEDGMENTS
All authors are current or former employees of Pfizer, Inc. We thank JANSSEN Alzheimer Immunotherapy (South San Francisco, CA) for their contribution to this study. We also thank Mike Agostino and Michelle Mader for help with sequence alignment, Chris Shea and Nicole Duriga for help with bionalytical assays, and Andrew Hill and Ioannis Moutsatsos for help with protein bioinformatics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vugmeyster, Y., Szklut, P., Wensel, D. et al. Complex Pharmacokinetics of a Humanized Antibody Against Human Amyloid Beta Peptide, Anti-Abeta Ab2, in Nonclinical Species. Pharm Res 28, 1696–1706 (2011). https://doi.org/10.1007/s11095-011-0405-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-011-0405-x