ABSTRACT
Purpose
The objective of this study is to develop a physiologically-based pharmacokinetic (PBPK) model for each omeprazole enantiomer that accounts for nonlinear PK of the two enantiomers as well as omeprazole racemic drug.
Methods
By integrating in vitro, in silico and human PK data, we first developed PBPK models for each enantiomer. Simulation of racemic omeprazole PK was accomplished by combining enantiomer models that allow mutual drug interactions to occur.
Results
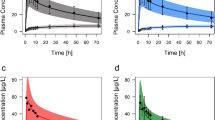
The established PBPK models for the first time satisfactorily predicted the nonlinear PK of esomeprazole, R-omeprazole and the racemic drug. The modeling exercises revealed that the strong time-dependent inhibition of CYP2C19 by esomeprazole greatly altered the R-omeprazole PK following administration of racemic omeprazole as in contrast to R-omeprazole given alone. When PBPK models incorporated both autoinhibition of each enantiomer and mutual interactions, the ratios between predicted and observed AUC following single and multiple dosing of omeprazole were 0.97 and 0.94, respectively.
Conclusions
PBPK models of omeprazole enantiomers and racemic drug were developed. These models can be utilized to assess CYP2C19-mediated drug and genetic interaction potential for omeprazole and esomeprazole.



Similar content being viewed by others
Abbreviations
- ADME:
-
Absorption, distribution, metabolism and excretion
- ASA:
-
Automated sensitivity analysis
- CLint :
-
Intrinsic clearance
- DDI:
-
Drug-drug interaction
- EM:
-
Extensive metabolizers
- HLM:
-
Human liver microsomes
- PBPK:
-
Physiologically based pharmacokinetic modeling
- PK:
-
Pharmacokinetics
- PM:
-
Poor metabolizers
- TDI:
-
Time-dependent inhibition
- Vss:
-
Volume of distribution at steady state
- WT:
-
Wild type
REFERENCES
Hassan-Alin M, Andersson T, Niazi M, Rohss K. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S-omeprazole (esomeprazole) and R-omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60(11):779–84.
Li XQ, Weidolf L, Simonsson R, Andersson TB. Enantiomer/enantiomer interactions between the S- and R- isomers of omeprazole in human cytochrome P450 enzymes: major role of CYP2C19 and CYP3A4. J Pharmacol Exp Ther. 2005;315(2):777–87.
Ogilvie BW, Yerino P, Kazmi F, Buckley DB, Rostami-Hodjegan A, Paris BL, et al. The proton pump inhibitor, omeprazole, but not lansoprazole or pantoprazole, is a metabolism-dependent inhibitor of CYP2C19: implications for coadministration with clopidogrel. Drug Metab Dispos. 2011;39(11):2020–33.
Andersson T, Weidolf L. Stereoselective disposition of proton pump inhibitors. Clin Drug Investig. 2008;28(5):263–79.
Furuta T, Shirai N, Sugimoto M, Nakamura A, Hishida A, Ishizaki T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet. 2005;20(3):153–67.
Hu XP, Xu JM, Hu YM, Mei Q, Xu XH. Effects of CYP2C19 genetic polymorphism on the pharmacokinetics and pharmacodynamics of omeprazole in Chinese people. J Clin Pharm Ther. 2007;32(5):517–24.
Foster DJ, Somogyi AA, White JM, Bochner F. Population pharmacokinetics of (R)-, (S)- and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2004;57(6):742–55.
Johansson CC, Gennemark P, Artursson P, Abelo A, Ashton M, Jansson-Lofmark R. Population pharmacokinetic modeling and deconvolution of enantioselective absorption of eflornithine in the rat. J Pharmacokinet Pharmacodyn. 2013;40(1):117–28.
Huang SM. PBPK as a tool in regulatory review. Biopharm Drug Dispos. 2012;33(2):51–2.
Huang SM, Rowland M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clin Pharmacol Ther. 2012;91(3):542–9.
Hassan-Alin M, Andersson T, Bredberg E, Rohss K. Pharmacokinetics of esomeprazole after oral and intravenous administration of single and repeated doses to healthy subjects. Eur J Clin Pharmacol. 2000;56(9–10):665–70.
Andersson T, Hassan-Alin M, Hasselgren G, Rohss K, Weidolf L. Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet. 2001;40(6):411–26.
Vieira ML, Zhao P, Berglund EG, Reynolds KS, Zhang L, Lesko LJ, et al. Predicting drug interaction potential with a physiologically based pharmacokinetic model: a case study of telithromycin, a time-dependent CYP3A inhibitor. Clin Pharmacol Ther. 2012;91(4):700–8.
Yang J, Liao M, Shou M, Jamei M, Yeo KR, Tucker GT, et al. Cytochrome p450 turnover: regulation of synthesis and degradation, methods for determining rates, and implications for the prediction of drug interactions. Curr Drug Metab. 2008;9(5):384–94.
DRUGS@FDA, Clinical pharmacology review; http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21154_nexium_biopharmr_p1.pdf. Last accessed July 12, 2013.
Uno T, Niioka T, Hayakari M, Yasui-Furukori N, Sugawara K, Tateishi T. Absolute bioavailability and metabolism of omeprazole in relation to CYP2C19 genotypes following single intravenous and oral administrations. Eur J Clin Pharmacol. 2007;63(2):143–9.
Sun SY, Wang YQ, Li LP, Wang L, Zeng S, Zhou H, et al. Stereoselective interaction between tetrahydropalmatine enantiomers and CYP enzymes in human liver microsomes. Chirality. 2013;25(1):43–7.
Tornio A, Niemi M, Neuvonen PJ, Backman JT. Stereoselective interaction between the CYP2C8 inhibitor gemfibrozil and racemic ibuprofen. Eur J Clin Pharmacol. 2007;63(5):463–9.
Sinko G, Kovarik Z, Reiner E, Simeon-Rudolf V, Stojan J. Mechanism of stereoselective interaction between butyrylcholinesterase and ethopropazine enantiomers. Biochimie. 2011;93(10):1797–807.
Markowitz JS, Patrick KS. Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter? J Clin Psychopharmacol. 2008;28(3 Suppl 2):S54–61.
Wainer IW, Granvil CP, Wang T, Batist G. Efficacy and toxicity of ifosfamide stereoisomers in an in vivo rat mammary carcinoma model. Cancer Res. 1994;54(16):4393–7.
Vakily M, Mehvar R, Brocks D. Stereoselective pharmacokinetics and pharmacodynamics of anti-asthma agents. Ann Pharmacother. 2002;36(4):693–701.
Walle T, Walle UK, Thornburg KR, Schey KL. Stereoselective sulfation of albuterol in humans. Biosynthesis of the sulfate conjugate by HEP G2 cells. Drug Metab Dispos. 1993;21(1):76–80.
Abelo A, Andersson TB, Antonsson M, Naudot AK, Skanberg I, Weidolf L. Stereoselective metabolism of omeprazole by human cytochrome P450 enzymes. Drug Metab Dispos. 2000;28(8):966–72.
Shirasaka Y, Sager JE, Lutz JD, Davis C, Isoherranen N. Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions. Drug Metab Dispos. 2013.
Furuta T, Ohashi K, Kamata T, Takashima M, Kosuge K, Kawasaki T, et al. Effect of genetic differences in omeprazole metabolism on cure rates for Helicobacter pylori infection and peptic ulcer. Ann Intern Med. 1998;129(12):1027–30.
Furuta T, Sagehashi Y, Shirai N, Sugimoto M, Nakamura A, Kodaira M, et al. Influence of CYP2C19 polymorphism and Helicobacter pylori genotype determined from gastric tissue samples on response to triple therapy for H pylori infection. Clin Gastroenterol Hepatol. 2005;3(6):564–73.
Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15(12):1929–37.
Kita T, Sakaeda T, Aoyama N, Sakai T, Kawahara Y, Kasuga M, et al. Optimal dose of omeprazole for CYP2C19 extensive metabolizers in anti-Helicobacter pylori therapy: pharmacokinetic considerations. Biol Pharm Bull. 2002;25(7):923–7.
Leong R, Vieira ML, Zhao P, Mulugeta Y, Lee CS, Huang SM, et al. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin Pharmacol Ther. 2012;91(5):926–31.
Grillo JA, Zhao P, Bullock J, Booth BP, Lu M, Robie-Suh K, et al. Utility of a physiologically-based pharmacokinetic (PBPK) modeling approach to quantitatively predict a complex drug-drug-disease interaction scenario for rivaroxaban during the drug review process: implications for clinical practice. Biopharm Drug Dispos. 2012;33(2):99–110.
Zhao P, Vieira ML, Grillo JA, Song P, Wu TC, Zheng JH, et al. Evaluation of exposure change of nonrenally eliminated drugs in patients with chronic kidney disease using physiologically based pharmacokinetic modeling and simulation. J Clin Pharmacol. 2012;52(1 Suppl):91S–108S.
Kang BC, Yang CQ, Cho HK, Suh OK, Shin WG. Influence of fluconazole on the pharmacokinetics of omeprazole in healthy volunteers. Biopharm Drug Dispos. 2002;23(2):77–81.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors would like to thank Professor Amin Rostami-Hodjegan from the University of Manchester for his valuable scientific input. This project was supported by FDA’s Critical Path Fellowship. This project was also supported in part by an appointment to the ORISE Research Participation Program at the Center for Drug Evaluation and Research administered by the Oak Ridge Institute for Science and Education through an agreement between the U.S. Department of Energy and CDER. The views presented in this manuscript are those of authors and do not necessarily reflect the official view of the FDA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, F., Gaohua, L., Zhao, P. et al. Predicting Nonlinear Pharmacokinetics of Omeprazole Enantiomers and Racemic Drug Using Physiologically Based Pharmacokinetic Modeling and Simulation: Application to Predict Drug/Genetic Interactions. Pharm Res 31, 1919–1929 (2014). https://doi.org/10.1007/s11095-013-1293-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1293-z