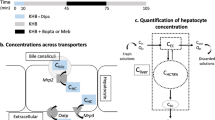
Abstract
Background and Objectives
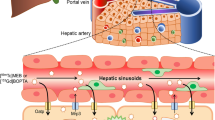
Gadobenate dimeglumine (Gd-BOPTA) is a commercialised hepatobiliary contrast agent used during liver magnetic resonance imaging (MRI) to detect liver diseases. It enters into human hepatocytes through organic anion transporting polypeptides (OATP1B1/B3) and crosses the canalicular transporter multiple resistance-associated protein 2 (MRP2) to be excreted into bile canaliculi. Gd-BOPTA can return to sinusoids via the sinusoidal transporters MRP3/MRP4. Hepatocyte concentrations of Gd-BOPTA depend on three clearances: the sinusoidal clearance or volume of sinusoidal blood cleared of drugs per unit of time and two hepatocyte clearances (into bile canaliculi or back to sinusoids) or volume of hepatocytes cleared of drugs per unit of time in the respective liver compartments. The present study investigates whether changing liver blood flow modifies hepatocyte concentrations when plasma concentrations do not change.
Methods
We perfused normal rat livers at various portal flow rates (24, 30, and 36 ml/min) with 200 µM Gd-BOPTA and measured sinusoidal clearances, hepatocyte clearances, and hepatocyte concentrations of Gd-BOPTA.
Results
We showed that varying portal flow rates changes the sinusoidal clearance of Gd-BOPTA despite its low extraction ratio. Portal flow rates do not modify Gd-BOPTA clearance from hepatocytes into bile canaliculi but can change hepatocyte clearance back to sinusoids.
Conclusion
At a given perfused concentration, portal flow rates modify Gd-BOPTA hepatocyte concentrations, a result important to consider when interpreting liver imaging.






Similar content being viewed by others
References
Paumgartner G. Biliary physiology and disease: reflections of a physician-scientist. Hepatology. 2010;51:1095–106.
Pastor CM, Müllhaupt B, Stieger B. The role of organic anion transporters in diagnosing liver diseases by magnetic resonance imaging. Drug Metab Dispos. 2014;42:675–84.
Leonhardt M, Keiser M, Oswald S, Kühn J, Jia J, Grube M, et al. Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos. 2010;38:1024–8.
Nassif A, Jia J, Keiser M, Oswald S, Modess C, Nagel S, et al. Visualization of hepatic uptake transporter function in healthy subjects by using gadoxetic acid-enhanced MR imaging. Radiology. 2012;264:741–50.
Jia J, Puls D, Oswald S, Jedlitschky G, Kühn JP, Weitschies W, et al. Characterization of the intestinal and hepatic uptake/efflux transport of the magnetic resonance imaging contrast agent gadolinium-ethoxylbenzyl-diethylenetriamine-pentaacetic acid. Invest Radiol. 2014;49:78–86.
Daali Y, Millet P, Dayer P, Pastor CM. Evidence of drug-drug interactions through uptake and efflux transport systems in rat hepatocytes: implications for cellular concentrations of competing drugs. Drug Metab Dispos. 2013;41:1548–56.
Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259–67.
Planchamp C, Beyer GJ, Slosman DO, Terrier F, Pastor CM. Direct evidence of the temperature dependence of Gd-BOPTA transport in the intact rat liver. Appl Radiat Isotopes. 2005;62:943–9.
Pastor CM. How transfer rates generate Gd-BOPTA concentrations in rat liver compartments: implications for clinical liver imaging with hepatobiliary contrast agents. Contrast Media Mol Imaging. 2016;11:291–8.
Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441–55.
Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035–78.
Klabunde RE. Cardiovascular physiology concepts. http://www.cvphysiology.com/Microcirculation/M016.htm. Assessed on October 2, 2016.
Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–339.
Fraser R, Bowler LM, Day WA, Dobbs B, Johnson HD, Lee D. High perfusion pressure damages the sieving ability of sinusoidal endothelium in rat livers. Br J Exp Pathol. 1980;61:222–8.
Nopanitaya W, Lamb JC, Grisham JW, Carson JL. Effect of hepatic venous outflow obstruction on pores and fenestration in sinusoidal endothelium. Br J Exp Pathol. 1976;57:604–9.
Liu L, Pang KS. An integrated approach to model hepatic drug clearance. Eur J Pharm Sci. 2006;29:215–30.
Chu X, Korzekwa K, Elsby R, Fenner K, Galetin A, Lai Y, et al. Intracellular drug concentrations and transporters: measurement, modeling, and implications for the liver. Clin Pharmacol Ther. 2013;94:126–41.
Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34:351–9.
Vander Borght S, Libbrecht L, Blokzijl H, Faber KN, Moshage H, Aerts R, et al. Diagnostic and pathogenetic implications of the expression of hepatic transporters in focal lesions occurring in normal liver. J Pathol. 2005;207:471–82.
Yoneda N, Matsui O, Kitao A, Kozaka K, Gabata T, Sasaki M, et al. Beta-catenin-activated hepatocellular adenoma showing hyperintensity on hepatobiliary-phase gadoxetic-enhanced magnetic resonance imaging and overexpression of OATP8. Jpn J Radiol. 2012;30:777–82.
Van Beers BE, Pastor CM, Hussain HK. Primovist, Eovist: what to expect? J Hepatol. 2012;57:421–9.
Brouwer KL, Keppler D, Hoffmaster KA, Bow DA, Cheng Y, Lai Y, et al. In vitro methods to support transporter evaluation in drug discovery and development. Clin Pharmacol Ther. 2013;94:95–112.
Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, et al. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging–correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817–26.
Tsuboyama T, Onishi H, Kim T, Akita H, Hori M, Tatsumi M, et al. Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR Imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology. 2010;255:824–33.
Vilgrain V, Van Beers BE, Pastor CM. Insights into the diagnosis of hepatocellular carcinomas with hepatobiliary MRI. J Hepatol. 2016;64:708–16.
Pastor CM, Morel DR, Billiar TR. Oxygen supply dependence of urea production in the isolated perfused rat liver. Am J Resp Crit Care Med. 1998;157:796–802.
Bonnaventure P, Pastor CM. Quantification of drug transport function across the multiple resistance-associated protein 2 (Mrp2) in rat livers. Int J Mol Sci. 2015;16:135–47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Swiss National Foundation (Grant N° 126030).
Conflict of interest
Jean-Luc Daire, Benjamin Leporq, Valérie Vilgrain, Bernard E Van Beers, Sabine Schmidt, and Catherine M Pastor have no conflicts of interest to declare.
Ethical approval
Study approved by the Geneva animal welfare committee and veterinary office. All institutional and national guidelines for the care of the laboratory animals were followed.
Rights and permissions
About this article
Cite this article
Daire, JL., Leporq, B., Vilgrain, V. et al. Liver Perfusion Modifies Gd-DTPA and Gd-BOPTA Hepatocyte Concentrations Through Transfer Clearances Across Sinusoidal Membranes. Eur J Drug Metab Pharmacokinet 42, 657–667 (2017). https://doi.org/10.1007/s13318-016-0382-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-016-0382-x