Abstract
Prednisolone and prednisone are integral components of induction and maintenance immunosuppressive regimens in solid organ transplantation. The pharmacokinetics of these agents are extremely complex. Prednisolone is the active drug moiety while prednisone is both a pro-drug and inactive metabolite of prednisolone. Within the dosage range used in transplantation, prednisolone and prednisone exhibit concentration-dependent non-linear pharmacokinetics when parameters are measured with reference to total drug concentration. Dose dependency disappears when free (unbound) prednisolone is measured. Altered organ function, changing biochemistry and use of a number of concomitant medicines in transplantation appear to lead to pharmacokinetic differences in transplant recipients compared with other patient groups. Greater than threefold variability in dose-adjusted exposure to total prednisolone in transplant recipients is evident. Time post-transplant, hepatic and renal dysfunction, patient age, sex, bodyweight, serum albumin concentration, concomitant medication exposure, various disease states and genetic polymorphisms in metabolic enzymes and drug transporters have sometimes been associated with prednisolone pharmacokinetic variability. The clinical impact of corticosteroid therapy on the disposition of ciclosporin, tacrolimus and sirolimus and the impact of different immunosuppressant therapy combinations on prednisolone exposure needs to be further elucidated. Patient response patterns to prednisolone are consistent with delayed and indirect mechanisms of corticosteroid action involving modification of nuclear transcription and protein synthesis. Many adverse effects have been linked with prednisolone and prednisone therapy, but not all of these have been investigated thoroughly in transplant populations. Dyslipidaemia, growth restriction, diabetogenesis, hypertension and cataracts are well studied toxicities. Evidence is less clear for prednisolone-induced osteonecrosis, obesity and hypertriglyceridaemia. There have been some reports of a relationship between prednisolone pharmacokinetics and incidence of acute rejection, Cushing’s syndrome and adverse cardiovascular and metabolic events. Dosing of prednisolone and prednisone in transplantation is typically empirical and varies significantly across transplant centres. Currently, authoritative guidelines are conflicting in their opinions regarding corticosteroid avoidance and early discontinuation in adult kidney transplantation. Overall, data suggest the promise of corticosteroid-free immunosuppression in paediatric patients. Further investigation of the pharmacokinetics and pharmacodynamics of prednisolone and prednisone in transplant recipients based on new chromatography assay techniques and free drug measurement, population pharmacokinetic/pharmacodynamic modelling approaches, genetic testing and larger studies in patients on modern day immunosuppressant protocols may lead to better individualization of corticosteroid therapy in the future.

Similar content being viewed by others
References
Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients: Transplant Data 1999–2009. Rockville, MD: U.S. Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation, Rockville, MD; United Network for Organ Sharing, Richmond, VA; University Renal Research and Education Association, Ann Arbor, MI; 2010. http://optn.transplant.hrsa.gov/ar2009/. Accessed 22 Feb 2012.
Pickup ME. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1979;4(2):111–28.
Czock D, Keller F, Rasche FM, et al. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61–98.
Diederich S, Eigendorff E, Burkhardt P, et al. 11Beta-hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab. 2002;87(12):5695–701.
Lexi-Comp Online, Hudson, Ohio. In: Basow D (ed). UpToDate. Waltham: UpToDate; 2012. http://www.uptodate.com. Accessed 20 Feb 2012.
MIMS Online [Internet database]. MIMS Australia; 2012. http://www.mims.com.au/index.php. Accessed 20 Feb 2012.
Gambertoglio JG, Frey FJ, Holford NH, et al. Prednisone and prednisolone bioavailability in renal transplant patients. Kidney Int. 1982;21(4):621–6.
Gambertoglio JG, Vincenti F, Feduska NJ, et al. Prednisolone disposition in cushingoid and noncushingoid kidney transplant patients. J Clin Endocrinol Metab. 1980;51(3):561–5.
Frey FJ, Schnetzer A, Horber FF, et al. Evidence that cyclosporine does not affect the metabolism of prednisolone after renal transplantation. Transplantation. 1987;43(4):494–8.
Garg V, Jusko WJ. Bioavailability and reversible metabolism of prednisone and prednisolone in man. Biopharm Drug Dispos. 1994;15(2):163–72.
Madsbad S, Bjerregaard B, Henriksen JH, et al. Impaired conversion of prednisone to prednisolone in patients with liver cirrhosis. Gut. 1980;21(1):52–6.
Renner E, Horber FF, Jost G, et al. Effect of liver function on the metabolism of prednisone and prednisolone in humans. Gastroenterology. 1986;90(4):819–28.
Frey BM, Frey FJ. Clinical pharmacokinetics of prednisone and prednisolone. Clin Pharmacokinet. 1990;19(2):126–46.
Karssen AM, Meijer OC, van der Sandt IC, et al. The role of the efflux transporter P-glycoprotein in brain penetration of prednisolone. J Endocrinol. 2002;175(1):251–60.
van Runnard Heimel PJ, Schobben AF, Huisjes AJ, et al. The transplacental passage of prednisolone in pregnancies complicated by early-onset HELLP syndrome. Placenta. 2005;26 (10):842–5.
Murphy VE, Fittock RJ, Zarzycki PK, et al. Metabolism of synthetic steroids by the human placenta. Placenta. 2007;28(1):39–46.
Beitins IZ, Bayard F, Ances IG, et al. The transplacental passage of prednisone and prednisolone in pregnancy near term. J Pediatr. 1972;81(5):936–45.
Ost L, Wettrell G, Bjorkhem I, et al. Prednisolone excretion in human milk. J Pediatr. 1985;106(6):1008–11.
Wald JA, Law RM, Ludwig EA, et al. Evaluation of dose-related pharmacokinetics and pharmacodynamics of prednisolone in man. J Pharmacokinet Biopharm. 1992;20(6):567–89.
Barth J, Damoiseaux M, Mollmann H, et al. Pharmacokinetics and pharmacodynamics of prednisolone after intravenous and oral administration. Int J Clin Pharmacol Ther Toxicol. 1992;30(9):317–24.
Xu J, Winkler J, Derendorf H. A pharmacokinetic/pharmacodynamic approach to predict total prednisolone concentrations in human plasma. J Pharmacokinet Pharmacodyn. 2007;34(3):355–72.
Vogt M, Derendorf H, Kramer J, et al. Biowaiver monographs for immediate release solid oral dosage forms: prednisolone. J Pharm Sci. 2007;96(1):27–37.
Rose JQ, Yurchak AM, Jusko WJ. Dose dependent pharmacokinetics of prednisone and prednisolone in man. J Pharmacokinet Biopharm. 1981;9(4):389–417.
Rohatagi S, Barth J, Mollmann H, et al. Pharmacokinetics of methylprednisolone and prednisolone after single and multiple oral administration. J Clin Pharmacol. 1997;37(10):916–25.
Rocci ML Jr, Szefler SJ, Acara M, et al. Prednisolone metabolism and excretion in the isolated perfused rat kidney. Drug Metab Dispos. 1981;9(3):177–82.
Pichard L, Fabre I, Daujat M, et al. Effect of corticosteroids on the expression of cytochromes P450 and on cyclosporin A oxidase activity in primary cultures of human hepatocytes. Mol Pharmacol. 1992;41(6):1047–55.
Ged C, Rouillon JM, Pichard L, et al. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989;28(4):373–87.
Wrighton SA, Brian WR, Sari MA, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol Pharmacol. 1990;38(2):207–13.
Penzak SR, Formentini E, Alfaro RM, et al. Prednisolone pharmacokinetics in the presence and absence of ritonavir after oral prednisone administration to healthy volunteers. J Acquir Immune Defic Syndr. 2005;40(5):573–80.
Garg V, Jusko WJ. Simultaneous analysis of prednisone, prednisolone and their major hydroxylated metabolites in urine by high-performance liquid chromatography. J Chromatogr. 1991;567(1):39–47.
Jusko WJ, Rose JQ. Monitoring prednisone and prednisolone. Ther Drug Monit. 1980;2(2):169–76.
Frey FJ, Ruegsegger MK, Frey BM. The dose-dependent systemic availability of prednisone: one reason for the reduced biological effect of alternate-day prednisone. Br J Clin Pharmacol. 1986;21(2):183–9.
Frey FJ, Gambertoglio JG, Frey BM, et al. Nonlinear plasma protein binding and haemodialysis clearance of prednisolone. Eur J Clin Pharmacol. 1982;23(1):65–74.
Jones J, Narwal S, Rosenbaum F, et al. Population pharmacokinetics of prednisolone in heart and lung transplant patients [abstract]. American Association of Pharmaceutical Scientists Annual Meeting. 2006. AASP Journal Vol 8(S2). http://www.aapsj.org/abstracts/AM_2006/AAPS2006-002918.pdf. Accessed 20 Aug 2012.
Jeng S, Chanchairujira T, Jusko W, et al. Prednisone metabolism in recipients of kidney or liver transplants and in lung recipients receiving ketoconazole. Transplantation. 2003;75(6):792–5.
Potter JM, McWhinney BC, Sampson L, et al. Area-under-the-curve monitoring of prednisolone for dose optimization in a stable renal transplant population. Ther Drug Monit. 2004;26(4):408–14.
Ost L. Impairment of prednisolone metabolism by cyclosporine treatment in renal graft recipients. Transplantation. 1987;44(4):533–5.
Ost L, Bjorkhem I, von Bahr C. Clinical value of assessing prednisolone pharmacokinetics before and after renal transplantation. Eur J Clin Pharmacol. 1984;26(3):363–9.
Uribe M, Schalm SW, Summerskill WH, et al. Oral prednisone for chronic active liver disease: dose responses and bioavailability studies. Gut. 1978;19(12):1131–5.
Schalm SW, Summerskill WH, Go VL. Prednisone for chronic active liver disease: pharmacokinetics, including conversion to prednisolone. Gastroenterology. 1977;72(5 Pt 1):910–3.
Kozower M, Veatch L, Kaplan MM. Decreased clearance of prednisolone, a factor in the development of corticosteroid side effects. J Clin Endocrinol Metab. 1974;38(3):407–12.
Davis M, Williams R, Chakraborty J, et al. Prednisone or prednisolone for the treatment of chronic active hepatitis? A comparison of plasma availability. Br J Clin Pharmacol. 1978;5(6):501–5.
Powell LW, Axelsen E. Corticosteroids in liver disease: studies on the biological conversion of prednisone to prednisolone and plasma protein binding. Gut. 1972;13(9):690–6.
Bergrem H. Pharmacokinetics and protein binding of prednisolone in patients with nephrotic syndrome and patients undergoing hemodialysis. Kidney Int. 1983;23(6):876–81.
Bergrem H. The influence of uremia on pharmacokinetics and protein binding of prednisolone. Acta Med Scand. 1983;213(5):333–7.
Kawai S, Ichikawa Y, Homma M. Differences in metabolic properties among cortisol, prednisolone, and dexamethasone in liver and renal diseases: accelerated metabolism of dexamethasone in renal failure. J Clin Endocrinol Metab. 1985;60(5):848–54.
Reece PA, Disney AP, Stafford I, et al. Prednisolone protein binding in renal transplant patients. Br J Clin Pharmacol. 1985;20(2):159–62.
Frey FJ. Pharmacokinetic determinants of cyclosporine and prednisone in renal transplant patients. Kidney Int. 1991;39(5):1034–50.
Leblond F, Guevin C, Demers C, et al. Downregulation of hepatic cytochrome P450 in chronic renal failure. J Am Soc Nephrol. 2001;12(2):326–32.
Benet LZ, Hoener BA. Changes in plasma protein binding have little clinical relevance. Clin Pharmacol Ther. 2002;71(3):115–21.
Gatti G, Perucca E, Frigo GM, et al. Pharmacokinetics of prednisone and its metabolite prednisolone in children with nephrotic syndrome during the active phase and in remission. Br J Clin Pharmacol. 1984;17(4):423–31.
Lewis GP, Jusko WJ, Graves L, et al. Prednisone side-effects and serum-protein levels: a collaborative study. Lancet. 1971;2(7728):778–80.
Uribe M, Summerskill W, Go V. Why hyperbilirubinemia and hypoalbuminemia pre-disposes to steroid side effects during treatment of chronic active liver disease. Gastroenterology. 1977;72:1143.
Stuck AE, Frey BM, Frey FJ. Kinetics of prednisolone and endogenous cortisol suppression in the elderly. Clin Pharmacol Ther. 1988;43(4):354–62.
Green OC, Winter RJ, Kawahara FS, et al. Plasma levels, half-life values, and correlation with physiologic assays for growth and immunity. J Pediatr. 1978;93(2):299–303.
Rose JQ, Nickelsen JA, Ellis EF, et al. Prednisolone disposition in steroid-dependent asthmatic children. J Allergy Clin Immunol. 1981;67(3):188–93.
Boekenoogen SJ, Szefler SJ, Jusko WJ. Prednisolone disposition and protein binding in oral contraceptive users. J Clin Endocrinol Metab. 1983;56(4):702.
Magee MH, Blum RA, Lates CD, et al. Prednisolone pharmacokinetics and pharmacodynamics in relation to sex and race. J Clin Pharmacol. 2001;41(11):1180–94.
Morton JM, Williamson S, Kear LM, et al. Therapeutic drug monitoring of prednisolone after lung transplantation. J Heart Lung Transplant. 2006;25(5):557–63.
Frey FJ, Frey BM. Urinary 6 beta-hydroxyprednisolone excretion indicates enhanced prednisolone catabolism. J Lab Clin Med. 1983;101(4):593–604.
Meffin PJ, Brooks PM, Sallustio BC. Alterations in prednisolone disposition as a result of time of administration, gender and dose. Br J Clin Pharmacol. 1984;17(4):395–404.
Milsap RL, Plaisance KI, Jusko WJ. Prednisolone disposition in obese men. Clin Pharmacol Ther. 1984;36(6):824–31.
Petersen KB, Jusko WJ, Rasmussen M, et al. Population pharmacokinetics of prednisolone in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2003;51(6):465–73.
Dove AM, Szefler SJ, Hill MR, et al. Altered prednisolone pharmacokinetics in patients with cystic fibrosis. J Pediatr. 1992;120(5):789–94.
Bergrem H, Opedal I. Bioavailability of prednisolone in patients with intestinal malabsorption: the importance of measuring serum protein-binding. Scand J Gastroenterol. 1983;18(4):545–9.
Elliott PR, Powell-Tuck J, Gillespie PE, et al. Prednisolone absorption in acute colitis. Gut. 1980;21(1):49–51.
Shaffer JA, Williams SE, Turnberg LA, et al. Absorption of prednisolone in patients with Crohn’s disease. Gut. 1983;24(3):182–6.
Milsap RL, George DE, Szefler SJ, et al. Effect of inflammatory bowel disease on absorption and disposition of prednisolone. Dig Dis Sci. 1983;28(2):161–8.
Pickup E, Record C, Farah F. Prednisolone absorption in patients with gastrointestinal disease. Ann Rheum Dis. 1978;37(6):566–76.
Pickup ME, Farah F, Lowe JR, et al. Prednisolone absorption in coeliac disease. Eur J Drug Metab Pharmacokinet. 1979;4(2):87–9.
Tanner AR, Halliday JW, Powell LW. Serum prednisolone levels in Crohn’s disease and coeliac disease following oral prednisolone administration. Digestion. 1981;21(6):310–5.
Frey FJ, Horber FF, Frey BM. Altered metabolism and decreased efficacy of prednisolone and prednisone in patients with hyperthyroidism. Clin Pharmacol Ther. 1988;44(5):510–21.
Bergamaschi S, Rusconi R, Gervasoni M, et al. Pharmacokinetics of prednisone and prednisolone in a case of hypothyroidism: effect of replacement therapy. Steroids. 2005;70(11):787–9.
Pichard L, Fabre I, Fabre G, et al. Cyclosporin A drug interactions: screening for inducers and inhibitors of cytochrome P-450 (cyclosporin A oxidase) in primary cultures of human hepatocytes and in liver microsomes. Drug Metab Dispos. 1990;18(5):595–606.
Yates CR, Chang C, Kearbey JD, et al. Structural determinants of P-glycoprotein-mediated transport of glucocorticoids. Pharm Res. 2003;20(11):1794–803.
Konishi H, Sumi M, Shibata N, et al. Decrease in oral bioavailability of ciclosporin by intravenous pulse of methylprednisolone succinate in rats. J Pharm Pharmacol. 2004;56(10):1259–66.
Lam S, Partovi N, Ting LS, et al. Corticosteroid interactions with cyclosporine, tacrolimus, mycophenolate, and sirolimus: fact or fiction? Ann Pharmacother. 2008;42(7):1037–47 (Epub 2008 Jul 1).
Griffin PJ, Da Costa CA, Salaman JR. A controlled trial of steroids in cyclosporine-treated renal transplant recipients. Transplantation. 1987;43(4):505–8.
Thiel G, Harder F, Loertscher R, et al. Cyclosporine alone or in combination with prednisone in cadaveric renal transplantation. Transplant Proc. 1984;16(5):1187–90.
Hricik DE, Moritz C, Mayes JT, et al. Association of the absence of steroid therapy with increased cyclosporine blood levels in renal transplant recipients. Transplantation. 1990;49(1):221–3.
Arnold JC, O’Grady JG, Tredger JM, et al. Effects of low-dose prednisolone on cyclosporine pharmacokinetics in liver transplant recipients: radioimmunoassay with specific and non-specific monoclonal antibodies. Eur J Clin Pharmacol. 1990;39(3):257–60.
Langhoff E, Madsen S, Olgaard K, et al. Clinical results and cyclosporine effect on prednisolone metabolism of cadaver kidney transplanted patients. Proc Eur Dial Transplant Assoc Eur Ren Assoc. 1984;21:963–8.
Ost L. Effects of cyclosporin on prednisolone metabolism. Lancet. 1984;1(8374):451.
Langhoff E, Madsen S, Flachs H, et al. Inhibition of prednisolone metabolism by cyclosporine in kidney-transplanted patients. Transplantation. 1985;39(1):107–9.
Undre NA, Schafer A. Factors affecting the pharmacokinetics of tacrolimus in the first year after renal transplantation: European Tacrolimus Multicentre Renal Study Group. Transplant Proc. 1998;30(4):1261–3.
van Duijnhoven EM, Boots JM, Christiaans MH, et al. Increase in tacrolimus trough levels after steroid withdrawal. Transpl Int. 2003;16(10):721–5.
Kim JS, Aviles DH, Silverstein DM, et al. Effect of age, ethnicity, and glucocorticoid use on tacrolimus pharmacokinetics in pediatric renal transplant patients. Pediatr Transplant. 2005;9(2):162–9.
Anglicheau D, Flamant M, Schlageter MH, et al. Pharmacokinetic interaction between corticosteroids and tacrolimus after renal transplantation. Nephrol Dial Transplant. 2003;18(11):2409–14.
Hesselink DA, Ngyuen H, Wabbijn M, et al. Tacrolimus dose requirement in renal transplant recipients is significantly higher when used in combination with corticosteroids. Br J Clin Pharmacol. 2003;56(3):327–30.
Park SI, Felipe CR, Pinheiro-Machado PG, et al. Tacrolimus pharmacokinetic drug interactions: effect of prednisone, mycophenolic acid or sirolimus. Fundam Clin Pharmacol. 2009;23(1):137–45.
Mourad M, Mourad G, Wallemacq P, et al. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation. 2005;80(7):977–84.
Cattaneo D, Merlini S, Pellegrino M, et al. Therapeutic drug monitoring of sirolimus: effect of concomitant immunosuppressive therapy and optimization of drug dosing. Am J Transplant. 2004;4(8):1345–51.
Jusko WJ, Ferron GM, Mis SM, et al. Pharmacokinetics of prednisolone during administration of sirolimus in patients with renal transplants. J Clin Pharmacol. 1996;36(12):1100–6.
Cattaneo D, Perico N, Gaspari F, et al. Glucocorticoids interfere with mycophenolate mofetil bioavailability in kidney transplantation. Kidney Int. 2002;62(3):1060–7.
Gregoor PJ, de Sevaux RG, Hene RJ, et al. Effect of cyclosporine on mycophenolic acid trough levels in kidney transplant recipients. Transplantation. 1999;68(10):1603–6.
van Hest RM, Mathot RA, Pescovitz MD, et al. Explaining variability in mycophenolic acid exposure to optimize mycophenolate mofetil dosing: a population pharmacokinetic meta-analysis of mycophenolic acid in renal transplant recipients. J Am Soc Nephrol. 2006;17(3):871–80.
Gambertoglio JG, Holford HG, Lizak PS, et al. The absence of effect of azathioprine on prednisolone pharmacokinetics following maintenance prednisone doses in kidney transplant patients. Am J Kidney Dis. 1984;3(6):425–9.
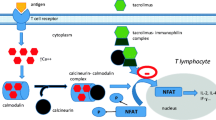
Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13.
Baxter JD, Forsham PH. Tissue effects of glucocorticoids. Am J Med. 1972;53(5):573–89.
Parrillo JE, Fauci AS. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19(1):179–201.
Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55(7):603–13.
Falkenstein E, Wehling M. Nongenomically initiated steroid actions. Eur J Clin Invest. 2000;30(Suppl 3):51–4.
Ferron GM, Jusko WJ. Species- and gender-related differences in cyclosporine/prednisolone/sirolimus interactions in whole blood lymphocyte proliferation assays. J Pharmacol Exp Ther. 1998;286(1):191–200.
Hong Y, Mager DE, Blum RA, et al. Population pharmacokinetic/pharmacodynamic modeling of systemic corticosteroid inhibition of whole blood lymphocytes: modeling interoccasion pharmacodynamic variability. Pharm Res. 2007;24(6):1088–97.
Wald JA, Jusko WJ. Prednisolone pharmacodynamics: leukocyte trafficking in the rat. Life Sci. 1994;55(19):PL371–8.
Morris HG. Factors that influence clinical responses to administered corticosteroids. J Allergy Clin Immunol. 1980;66(5):343–6.
Nouri-Majalan N, Sanadgol H, Rahimian M, et al. Bone mineral density in kidney transplant recipients and patients on hemodialysis: a comparison with healthy individuals. Iran J Kidney Dis. 2008;2(3):154–9.
Patel S, Kwan JT, McCloskey E, et al. Prevalence and causes of low bone density and fractures in kidney transplant patients. J Bone Miner Res. 2001;16(10):1863–70.
Goffin E, Devogelaer JP, Lalaoui A, et al. Tacrolimus and low-dose steroid immunosuppression preserves bone mass after renal transplantation. Transpl Int. 2002;15(2–3):73–80.
Yun YS, Kim BJ, Hong SP, et al. Changes of bone metabolism indices in patients receiving immunosuppressive therapy including low doses of steroids after renal transplantation. Transplant Proc. 1996;28(3):1561–4.
Martinez Diaz-Guerra G, Gomez R, Jodar E, et al. Long-term follow-up of bone mass after orthotopic liver transplantation: effect of steroid withdrawal from the immunosuppressive regimen. Osteoporos Int. 2002;13(2):147–50.
Gomez R, Moreno E, Colina F, et al. Steroid withdrawal is safe and beneficial in stable cyclosporine-treated liver transplant patients. J Hepatol. 1998;28(1):150–6.
Matas AJ, Kandaswamy R, Gillingham KJ, et al. Prednisone-free maintenance immunosuppression-a 5-year experience. Am J Transplant. 2005;5(10):2473–8.
Woodle ES, First MR, Pirsch J, et al. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248(4):564–77.
Vincenti F, Schena FP, Paraskevas S, et al. A randomized, multicenter study of steroid avoidance, early steroid withdrawal or standard steroid therapy in kidney transplant recipients. Am J Transplant. 2008;8(2):307–16.
Kasiske BL, Vazquez MA, Harmon WE, et al. Recommendations for the outpatient surveillance of renal transplant recipients: American Society of Transplantation. J Am Soc Nephrol. 2000;11(Suppl 15):S1–86.
Selby PL, Halsey JP, Adams KR, et al. Corticosteroids do not alter the threshold for vertebral fracture. J Bone Miner Res. 2000;15(5):952–6.
Weinstein RS. Clinical practice: glucocorticoid-induced bone disease. N Engl J Med. 2011;365(1):62–70.
Stein EM, Ortiz D, Jin Z, et al. Prevention of fractures after solid organ transplantation: a meta-analysis. J Clin Endocrinol Metab. 2011;96(11):3457–65.
Palmer SC, McGregor DO, Strippoli GF. Interventions for preventing bone disease in kidney transplant recipients. Cochrane Database Syst Rev 2007;(3):CD005015.
Lausten GS, Lemser T, Jensen PK, et al. Necrosis of the femoral head after kidney transplantation. Clin Transplant. 1998;12(6):572–4.
Kubo T, Fujioka M, Yamazoe S, et al. Relationship between steroid dosage and osteonecrosis of the femoral head after renal transplantation as measured by magnetic resonance imaging. Transplant Proc. 1998;30(7):3039–40.
Fryer JP, Benedetti E, Gillingham K, et al. Steroid-related complications in pediatric kidney transplant recipients in the cyclosporine era. Transplant Proc. 1994;26(1):91–2.
Inoue S, Horii M, Asano T, et al. Risk factors for nontraumatic osteonecrosis of the femoral head after renal transplantation. J Orthop Sci. 2003;8(6):751–6.
Salaman JR, Gomes Da Costa CA, Griffin PJ. Renal transplantation without steroids. J Pediatr. 1987;111(6 Pt 2):1026–8.
Dolgos S, Hartmann A, Jenssen T, et al. Determinants of short-term changes in body composition following renal transplantation. Scand J Urol Nephrol. 2009;43(1):76–83.
Hoy WE, Sargent JA, Freeman RB, et al. The influence of glucocorticoid dose on protein catabolism after renal transplantation. Am J Med Sci. 1986;291(4):241–7.
Nematalla AH, Bakr MA, Gheith OA, et al. Steroid-avoidance immunosuppression regimen in live-donor renal allotransplant recipients: a prospective, randomized, controlled study. Exp Clin Transplant. 2007;5(2):673–9.
Fabrega AJ, Cohan J, Meslar P, et al. Effects of steroid withdrawal on long-term renal allograft recipients with posttransplantation diabetes mellitus. Surgery. 1994;116(4):792–7.
Ko TY, Haddy JA, Marcus RJ, et al. Steroid avoidance in renal transplant patients maintained on a cyclosporine-based protocol. Exp Clin Transplant. 2007;5(2):664–9.
Gavela E, Avila A, Sancho A, et al. Benefits derivated from late steroid withdrawal in renal transplant recipients. Transplant Proc. 2007;39(7):2173–5.
Laube GF, Falger J, Kemper MJ, et al. Selective late steroid withdrawal after renal transplantation. Pediatr Nephrol. 2007;22(11):1947–52.
van den Ham EC, Kooman JP, Christiaans MH, et al. Relation between steroid dose, body composition and physical activity in renal transplant patients. Transplantation. 2000;69(8):1591–8.
Fabrega AJ, Meslar P, Cohan J, et al. Long-term (24-month) follow-up of steroid withdrawal in renal allograft recipients with posttransplant diabetes mellitus. Transplantation. 1995;60(12):1612–4.
Tisone G, Angelico M, Vennarecci G, et al. Metabolic findings after liver transplantation within a randomised trial with or without steroids. Transplant Proc. 1998;30(4):1447–8.
Singh A, Tejani A. Hyperlipidemia in children: the role of uremia, steroids and cyclosporine therapy. Nephron. 1996;74(3):529–35.
Ahsan N, Hricik D, Matas A, et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil: a prospective randomized study. Steroid Withdrawal Study Group. Transplantation. 1999;68(12):1865–74.
Vaziri ND, Risk C, Martin DC, et al. Comparison of hyperlipidemia in dialysis patients, renal transplant recipients, and steroid treated nonrenal patients. J Dial. 1980;4(2–3):63–71.
Ratcliffe PJ, Dudley CR, Higgins RM, et al. Randomised controlled trial of steroid withdrawal in renal transplant recipients receiving triple immunosuppression. Lancet. 1996;348(9028):643–8.
Laftavi MR, Stephan R, Stefanick B, et al. Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery. 2005;137(3):364–71.
Gulanikar AC, Belitsky P, MacDonald AS, et al. Randomized controlled trial of steroids versus no steroids in stable cyclosporine-treated renal graft recipients. Transplant Proc. 1991;23(1 Pt 2):990–1.
Capell HA, Madhok R, Hunter JA, et al. Lack of radiological and clinical benefit over two years of low dose prednisolone for rheumatoid arthritis: results of a randomised controlled trial. Ann Rheum Dis. 2004;63(7):797–803.
Pascual J, Galeano C, Royuela A, et al. A systematic review on steroid withdrawal between 3 and 6 months after kidney transplantation. Transplantation. 2010;90(4):343–9.
Pascual J, Quereda C, Zamora J, et al. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: a meta-analysis of randomized, controlled trials. Transplantation. 2004;78(10):1548–56.
Li L, Chang A, Naesens M, et al. Steroid-free immunosuppression since 1999: 129 pediatric renal transplants with sustained graft and patient benefits. Am J Transplant. 2009;9(6):1362–72.
Vanrenterghem Y, Lebranchu Y, Hene R, et al. Double-blind comparison of two corticosteroid regimens plus mycophenolate mofetil and cyclosporine for prevention of acute renal allograft rejection. Transplantation. 2000;70(9):1352–9.
Arner P, Gunnarsson R, Blomdahl S, et al. Some characteristics of steroid diabetes: a study in renal-transplant recipients receiving high-dose corticosteroid therapy. Diabetes Care. 1983;6(1):23–5.
Boots JM, Christiaans MH, Van Duijnhoven EM, et al. Early steroid withdrawal in renal transplantation with tacrolimus dual therapy: a pilot study. Transplantation. 2002;74(12):1703–9.
Gurwitz JH, Bohn RL, Glynn RJ, et al. Glucocorticoids and the risk for initiation of hypoglycemic therapy. Arch Intern Med. 1994;154(1):97–101.
Hjelmesaeth J, Midtvedt K, Jenssen T, et al. Insulin resistance after renal transplantation: impact of immunosuppressive and antihypertensive therapy. Diabetes Care. 2001;24(12):2121–6.
Luan FL, Steffick DE, Ojo AO. New-onset diabetes mellitus in kidney transplant recipients discharged on steroid-free immunosuppression. Transplantation. 2011;91(3):334–41.
Bergrem HA, Valderhaug TG, Hartmann A, et al. Glucose tolerance before and after renal transplantation. Nephrol Dial Transplant. 2010;25(3):985–92.
Hjelmesaeth J, Hartmann A, Kofstad J, et al. Tapering off prednisolone and cyclosporin the first year after renal transplantation: the effect on glucose tolerance. Nephrol Dial Transplant. 2001;16(4):829–35.
Taler SJ, Textor SC, Canzanello VJ, et al. Role of steroid dose in hypertension early after liver transplantation with tacrolimus (FK506) and cyclosporine. Transplantation. 1996;62(11):1588–92.
Fine RN, Korsch BM, Brennan LP, et al. Renal transplantation in young children. Am J Surg. 1973;125(5):559–69.
McHugh MI, Tanboga H, Wilkinson R. Alternate day steroids and blood pressure control after renal transplantation. Proc Eur Dial Transplant Assoc. 1980;17:496–501.
Fellstrom B. Risk factors for and management of post-transplantation cardiovascular disease. BioDrugs. 2001;15(4):261–78.
Maxwell SR, Moots RJ, Kendall MJ. Corticosteroids: do they damage the cardiovascular system? Postgrad Med J. 1994;70(830):863–70.
Kramer BK, Klinger M, Wlodarczyk Z, et al. Tacrolimus combined with two different corticosteroid-free regimens compared with a standard triple regimen in renal transplantation: one year observational results. Clin Transplant 2010;24(1):E1–9.
Fryer AA, Ramsay HM, Lovatt TJ, et al. Polymorphisms in glutathione S-transferases and non-melanoma skin cancer risk in Australian renal transplant recipients. Carcinogenesis. 2005;26(1):185–91.
Urban RC, Jr., Cotlier E. Corticosteroid-induced cataracts. Surv Ophthalmol. 1986;31(2):102–10.
Fryer JP, Granger DK, Leventhal JR, et al. Steroid-related complications in the cyclosporine era. Clin Transplant. 1994;8(3 Pt 1):224–9.
Matsunami C, Hilton AF, Dyer JA, et al. Ocular complications in renal transplant patients. Aust N Z J Ophthalmol. 1994;22(1):53–7.
Grenda R, Watson A, Trompeter R, et al. A randomized trial to assess the impact of early steroid withdrawal on growth in pediatric renal transplantation: the TWIST study. Am J Transplant. 2010;10(4):828–36.
Hovland KR, Ellis PP. Ocular changes in renal transplant patients. Am J Ophthalmol. 1967;63(2):283–9.
Kian-Ersi F, Taheri S, Akhlaghi MR. Ocular disorders in renal transplant patients. Saudi J Kidney Dis Transpl. 2008;19(5):751–5.
Seydoux C, Berguer DG, Stumpe F, et al. Does early steroid withdrawal influence rejection and infection episodes during the first 2 years after heart transplantation? Transplant Proc. 1997;29(1–2):620–4.
Pelletier SJ, Vanderwall K, Debroy MA, et al. Preliminary analysis of early outcomes of a prospective, randomized trial of complete steroid avoidance in liver transplantation. Transplant Proc. 2005;37(2):1214–6.
Tan JY, Zhao N, Wu TX, et al. Steroid withdrawal increases risk of acute rejection but reduces infection: a meta-analysis of 1681 cases in renal transplantation. Transplant Proc. 2006;38(7):2054–6.
Tanchanco R, Krishnamurthi V, Winans C, et al. Beneficial outcomes of a steroid-free regimen with thymoglobulin induction in pancreas-kidney transplantation. Transplant Proc. 2008;40(5):1551–4.
Rees L, Chantler C. Growth and endocrine function in children receiving long-term steroid therapy for renal disease. Acta Paediatr Scand Suppl. 1990;366:93–6 (discussion 7).
Grenda R. Minimizing steroid use in pediatric kidney recipients. Pediatr Transplant. 2011;15(1):32–6.
Jabs K, Sullivan EK, Avner ED, et al. Alternate-day steroid dosing improves growth without adversely affecting graft survival or long-term graft function: a report of the North American Pediatric Renal Transplant Cooperative Study. Transplantation. 1996;61(1):31–6.
Potter DE, Holliday MA, Wilson CJ, et al. Alternate-day steroids in children after renal transplantation. Transplant Proc. 1975;7(1):79–82.
Grenda R. Effects of steroid avoidance and novel protocols on growth in paediatric renal transplant patients. Pediatr Nephrol. 2010;25(4):747–52.
Vidhun JR, Sarwal MM. Corticosteroid avoidance in pediatric renal transplantation: can it be achieved? Paediatr Drugs. 2004;6(5):273–87.
KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155.
Keller F, Hemmen T, Schoneshofer M, et al. Pharmacokinetics of methylprednisolone and rejection episodes in kidney transplant patients. Transplantation. 1995;60(4):330–3.
Bergrem H, Jervell J, Flatmark A. Prednisolone pharmacokinetics in cushingoid and non-cushingoid kidney transplant patients. Kidney Int. 1985;27(2):459–64.
Kang CM, Ahn JH, Kahng KW, et al. Pharmacokinetic characteristics of methylprednisolone in Korean renal transplant recipients. Transplant Proc. 1999;31(7):2759–60.
Frey FJ, Amend WJ, Lozada F, et al. Pharmacokinetics of prednisolone and endogenous hydrocortisone levels in cushingoid and non-cushingoid patients. Eur J Clin Pharmacol. 1981;21(3):235–42.
Benet LZ, Frey FJ, Amend WJ Jr, et al. Endogenous and exogenous glucocorticoids in cushingoid patients. Drug Intell Clin Pharm. 1982;16(11):863–8.
Sugioka N, Kokuhu T, Okamoto M, et al. Effect of plasma lipid on pharmacokinetics of ciclosporin and its relationship with plasma prednisolone level in renal transplant patients. J Pharm Pharmacol. 2006;58(9):1193–200.
Bergrem HA, Bergrem H, Hartmann A, et al. Role of prednisolone pharmacokinetics in postchallenge glycemia after renal transplantation. Ther Drug Monit. 2008;30(5):583–90.
Pascussi JM, Gerbal-Chaloin S, Drocourt L, et al. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta. 2003;1619(3):243–53.
Rosenfeld JM, Vargas R Jr, Xie W, et al. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17(7):1268–82.
Miura M, Satoh S, Inoue K, et al. Influence of CYP3A5, ABCB1 and NR1I2 polymorphisms on prednisolone pharmacokinetics in renal transplant recipients. Steroids. 2008;73(11):1052–9.
Miura M, Inoue K, Kagaya H, et al. Inter-individual difference determinant of prednisolone pharmacokinetics for Japanese renal transplant recipients in the maintenance stage. Xenobiotica. 2009;39(12):939–45.
Zheng H, Webber S, Zeevi A, et al. The MDR1 polymorphisms at exons 21 and 26 predict steroid weaning in pediatric heart transplant patients. Hum Immunol. 2002;63(9):765–70.
Hirata T, Fujioka M, Takahashi KA, et al. ApoB C7623T polymorphism predicts risk for steroid-induced osteonecrosis of the femoral head after renal transplantation. J Orthop Sci. 2007;12(3):199–206.
Asano T, Takahashi KA, Fujioka M, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics. 2003;13(11):675–82.
Decker SO, Keller F, Mayer J, et al. Twice daily fractionated dose administration of prednisolone compared to standard once daily administration to patients with glomerulonephritis or with kidney transplants. Med Klin (Munich). 2009;104(6):429–33.
Broyer M, Guest G, Gagnadoux MF. Growth rate in children receiving alternate-day corticosteroid treatment after kidney transplantation. J Pediatr. 1992;120(5):721–5.
Helal I, Chan L. Steroid and calcineurin inhibitor-sparing protocols in kidney transplantation. Transplant Proc. 2011;43(2):472–7.
Pascual J, Zamora J, Galeano C, et al. Steroid avoidance or withdrawal for kidney transplant recipients. Cochrane Database Syst Rev. 2009;(1):CD005632.
Kumar MS, Xiao SG, Fyfe B, et al. Steroid avoidance in renal transplantation using basiliximab induction, cyclosporine-based immunosuppression and protocol biopsies. Clin Transplant. 2005;19(1):61–9.
Vincenti F, Monaco A, Grinyo J, et al. Multicenter randomized prospective trial of steroid withdrawal in renal transplant recipients receiving basiliximab, cyclosporine microemulsion and mycophenolate mofetil. Am J Transplant. 2003;3(3):306–11.
Montagnino G, Sandrini S, Casciani C, et al. A randomized trial of steroid avoidance in renal transplant patients treated with everolimus and cyclosporine. Transplant Proc. 2005;37(2):788–90.
Vitko S, Klinger M, Salmela K, et al. Two corticosteroid-free regimens-tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil-in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation. 2005;80(12):1734–41.
Chadban SJ, Barraclough KA, Campbell SB, et al. KHA-CARI guideline: KHA-CARI adaptation of the KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Nephrology (Carlton). 2012;17(3):204–14.
US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Table 1.9a: Immunosuppression Use by Organ in 2007 and 2008. http://www.ustransplant.org/annual_reports/current/109a_dh.htm. Accessed 20 Oct 2011.
Clayton P, Excell L, Campbell S, McDonald S, Chadban S. Australia and New Zealand Dialysis and Transplant Registry 2010 Report. Transplantation, Chapter 8. http://www.anzdata.org.au/anzdata/AnzdataReport/33rdReport/Ch08.pdf. Accessed 20 Oct 2011.
Miller LW, Wolford T, McBride LR, et al. Successful withdrawal of corticosteroids in heart transplantation. J Heart Lung Transplant. 1992;11(2 Pt 2):431–4.
Olivari MT, Jessen ME, Baldwin BJ, et al. Triple-drug immunosuppression with steroid discontinuation by six months after heart transplantation. J Heart Lung Transplant. 1995;14(1 Pt 1):127–35.
Kobashigawa JA, Stevenson LW, Brownfield ED, et al. Initial success of steroid weaning late after heart transplantation. J Heart Lung Transplant. 1992;11(2 Pt 2):428–30.
Teuteberg JJ, Shullo M, Zomak R, et al. Aggressive steroid weaning after cardiac transplantation is possible without the additional risk of significant rejection. Clin Transplant 2008; 22(6):730–7.
Yamani MH, Taylor DO, Czerr J, et al. Thymoglobulin induction and steroid avoidance in cardiac transplantation: results of a prospective, randomized, controlled study. Clin Transplant. 2008;22(1):76–81.
International Society of Heart and Lung Transplantation Guidelines for the Care of Heart Transplant Recipients 2010. http://www.ishlt.org/ContentDocuments/ISHLT_GL_TaskForce2_110810.pdf. Accessed 30 May 2012.
Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report–2011. J Heart Lung Transplant. 2011;30(10):1078–94.
Knoop C, Haverich A, Fischer S. Immunosuppressive therapy after human lung transplantation. Eur Respir J. 2004;23(1):159–71.
US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. Lung: recipients: immunosuppression use. http://www.ustransplant.org/annual_reports/current/iLU_Recipients_immuno.htm?o=6&g=2&c=16. Accessed 30 May 2012.
Henry SD, Metselaar HJ, Van Dijck J, et al. Impact of steroids on hepatitis C virus replication in vivo and in vitro. Ann N Y Acad Sci. 2007;1110:439–47.
Moench C, Barreiros AP, Schuchmann M, et al. Tacrolimus monotherapy without steroids after liver transplantation: a prospective randomized double-blinded placebo-controlled trial. Am J Transplant. 2007;7(6):1616–23.
Weiler N, Thrun I, Hoppe-Lotichius M, et al. Early steroid-free immunosuppression with FK506 after liver transplantation: long-term results of a prospectively randomized double-blinded trial. Transplantation. 2010;90(12):1562–6.
The American Association for the Study of Liver Diseases. Practice guidelines. 2012. http://www.aasld.org/practiceguidelines/pages/default.aspx. Accessed 16 Jun 2012.
Gastroenterology Society of Australia. GESA clinical updates and recommendations. http://www.gesa.org.au/professional.asp?cid=9&id=51. Accessed 16 Jun 2012.
Vidhun JR, Sarwal MM. Corticosteroid avoidance in pediatric renal transplantation. Pediatr Nephrol. 2005;20(3):418–26.
Barletta GM, Kirk E, Gardner JJ, et al. Rapid discontinuation of corticosteroids in pediatric renal transplantation. Pediatr Transplant. 2009;13(5):571–8.
Lau KK, Berg GM, Schjoneman YG, et al. Extended experience with a steroid minimization immunosuppression protocol in pediatric renal transplant recipients. Pediatr Transplant. 2010;14(4):488–95.
Sarwal MM, Vidhun JR, Alexander SR, et al. Continued superior outcomes with modification and lengthened follow-up of a steroid-avoidance pilot with extended daclizumab induction in pediatric renal transplantation. Transplantation. 2003;76(9):1331–9.
Hocker B, Weber LT, Feneberg R, et al. Prospective, randomized trial on late steroid withdrawal in pediatric renal transplant recipients under cyclosporine microemulsion and mycophenolate mofetil. Transplantation. 2009;87(6):934–41.
Hocker B, Weber LT, Feneberg R, et al. Improved growth and cardiovascular risk after late steroid withdrawal: 2-year results of a prospective, randomised trial in paediatric renal transplantation. Nephrol Dial Transplant. 2010;25(2):617–24.
Leonard HC, O’Sullivan JJ, Dark JH. Long-term follow-up of pediatric cardiac transplant recipients on a steroid-free regime: the role of endomyocardial biopsy. J Heart Lung Transplant. 2000;19(5):469–72.
Leonard H, Hornung T, Parry G, et al. Pediatric cardiac transplant: results using a steroid-free maintenance regimen. Pediatr Transplant. 2003;7(1):59–63.
Singh TP, Faber C, Blume ED, et al. Safety and early outcomes using a corticosteroid-avoidance immunosuppression protocol in pediatric heart transplant recipients. J Heart Lung Transplant. 2010;29(5):517–22.
Janusinfo. Lakemedel och fosterskada. Prednisolone. http://www.janusinfo.se/v/Lakemedel-och-fosterpaverkan/?docid=2052. Accessed 2 Feb 2012.
Briggs G, Freeman R, Yaffe S, editors. Drugs in pregnancy and lactation. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2011.
Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology. 2000;62(6):385–92.
Saito J, Davis J, Wasnich R, et al. Users of low-dose glucocorticoids have increased bone loss rates: a longitudinal study. Calcif Tissue Int. 1995;57(2):115–9.
McWhinney BC, Briscoe SE, Ungerer JP, et al. Measurement of cortisol, cortisone, prednisolone, dexamethasone and 11-deoxycortisol with ultra high performance liquid chromatography-tandem mass spectrometry: application for plasma, plasma ultrafiltrate, urine and saliva in a routine laboratory. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(28):2863–9.
Ionita IA, Fast DM, Akhlaghi F. Development of a sensitive and selective method for the quantitative analysis of cortisol, cortisone, prednisolone and prednisone in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(8–9):765–72.
Taylor RL, Grebe SK, Singh RJ. Quantitative, highly sensitive liquid chromatography-tandem mass spectrometry method for detection of synthetic corticosteroids. Clin Chem. 2004;50(12):2345–52.
Miura M, Satoh S, Kagaya H, et al. No impact of age on dose-adjusted pharmacokinetics of tacrolimus, mycophenolic acid and prednisolone 1 month after renal transplantation. Eur J Clin Pharmacol. 2009;65(10):1047–53.
Rocci ML Jr, Tietze KJ, Lee J, et al. The effect of cyclosporine on the pharmacokinetics of prednisolone in renal transplant patients. Transplantation. 1988;45(3):656–60.
Hollander AA, van Rooij J, Lentjes GW, et al. The effect of grapefruit juice on cyclosporine and prednisone metabolism in transplant patients. Clin Pharmacol Ther. 1995;57(3):318–24.
Frey BM, Seeberger M, Frey FJ. Pharmacokinetics of 3 prednisolone prodrugs: evidence of therapeutic inequivalence in renal transplant patients with rejection. Transplantation. 1985;39(3):270–4.
Chavatte C, Guest G, Proust V, et al. Glucocorticoid pharmacokinetics and growth retardation in children with renal transplants. Pediatr Nephrol. 2004;19(8):898–904.
Uribe M, Casian C, Rojas S, et al. Decreased bioavailability of prednisone due to antacids in patients with chronic active liver disease and in healthy volunteers. Gastroenterology. 1981;80(4):661–5.
Tanner AR, Caffin JA, Halliday JW, et al. Concurrent administration of antacids and prednisone: effect on serum levels of prednisolone. Br J Clin Pharmacol. 1979;7(4):397–400.
Lee DA, Taylor GM, Walker JG, et al. The effect of concurrent administration of antacids on prednisolone absorption. Br J Clin Pharmacol. 1979;8(1):92–4.
Bartoszek M, Brenner AM, Szefler SJ. Prednisolone and methylprednisolone kinetics in children receiving anticonvulsant therapy. Clin Pharmacol Ther. 1987;42(4):424–32.
Olivesi A. Modified elimination of prednisolone in epileptic patients on carbamazepine monotherapy, and in women using low-dose oral contraceptives. Biomed Pharmacother. 1986;40(8):301–8.
Zurcher RM, Frey BM, Frey FJ. Impact of ketoconazole on the metabolism of prednisolone. Clin Pharmacol Ther. 1989;45(4):366–72.
Varis T, Kivisto KT, Neuvonen PJ. The effect of itraconazole on the pharmacokinetics and pharmacodynamics of oral prednisolone. Eur J Clin Pharmacol. 2000;56(1):57–60.
Denning DW, Van Wye JE, Lewiston NJ, et al. Adjunctive therapy of allergic bronchopulmonary aspergillosis with itraconazole. Chest. 1991;100(3):813–9.
Legler UF. Impairment of prednisolone disposition in patients with Graves’ disease taking methimazole. J Clin Endocrinol Metab. 1988;66(1):221–3.
Sarma GR, Kailasam S, Nair NG, et al. Effect of prednisolone and rifampin on isoniazid metabolism in slow and rapid inactivators of isoniazid. Antimicrob Agents Chemother. 1980;18(5):661–6.
Klinenberg JR, Miller F. Effect of corticosteroids on blood salicylate concentration. JAMA. 1965;194(6):601–4.
Imani S, Jusko WJ, Steiner R. Diltiazem retards the metabolism of oral prednisone with effects on T-cell markers. Pediatr Transplant. 1999;3(2):126–30.
Finkenbine RD, Frye MD. Case of psychosis due to prednisone-clarithromycin interaction. Gen Hosp Psychiatry. 1998;20(5):325–6.
Finkenbine R, Gill HS. Case of mania due to prednisone-clarithromycin interaction. Can J Psychiatry. 1997;42(7):778.
Widmer P, Maibach R, Kunzi UP, et al. Diuretic-related hypokalaemia: the role of diuretics, potassium supplements, glucocorticoids and beta 2-adrenoceptor agonists. Results from the comprehensive hospital drug monitoring programme, berne (CHDM). Eur J Clin Pharmacol. 1995;49(1–2):31–6.
Ojima M, Satoh K, Gomibuchi T, et al. The inhibitory effects of glycyrrhizin and glycyrrhetinic acid on the metabolism of cortisol and prednisolone: in vivo and in vitro studies (in Japanese). Nihon Naibunpi Gakkai Zasshi. 1990;66(5):584–96.
Chen MF, Shimada F, Kato H, et al. Effect of oral administration of glycyrrhizin on the pharmacokinetics of prednisolone. Endocrinol Jpn. 1991;38(2):167–74.
Frey BM, Schaad HJ, Frey FJ. Pharmacokinetic interaction of contraceptive steroids with prednisone and prednisolone. Eur J Clin Pharmacol. 1984;26(4):505–11.
Legler UF, Benet LZ. Marked alterations in dose-dependent prednisolone kinetics in women taking oral contraceptives. Clin Pharmacol Ther. 1986;39(4):425–9.
Seidegard J, Simonsson M, Edsbacker S. Effect of an oral contraceptive on the plasma levels of budesonide and prednisolone and the influence on plasma cortisol. Clin Pharmacol Ther. 2000;67(4):373–81.
Department of Health. Immunisation against infectious disease 2006. In: Salisbury D, Ramsay M, Noakes K, editors. London: The Stationery Office; 2012.
Weil J, Langman MJ, Wainwright P, et al. Peptic ulcer bleeding: accessory risk factors and interactions with non-steroidal anti-inflammatory drugs. Gut. 2000;46(1):27–31.
Piper JM, Ray WA, Daugherty JR, et al. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;114(9):735–40.
Carson JL, Strom BL, Schinnar R, et al. The low risk of upper gastrointestinal bleeding in patients dispensed corticosteroids. Am J Med. 1991;91(3):223–8.
Rae SA, Williams IA, English J, et al. Alteration of plasma prednisolone levels by indomethacin and naproxen. Br J Clin Pharmacol. 1982;14(3):459–61.
Patial RK, Bansal SK, Kashyap S, et al. Drug interaction-induced osteonecrosis of femoral head. J Assoc Physicians India. 1990;38(6):446–7.
Busse KH, Formentini E, Alfaro RM, et al. Influence of antiretroviral drugs on the pharmacokinetics of prednisolone in HIV-infected individuals. J Acquir Immune Defic Syndr. 2008;48(5):561–6.
Acknowledgments
The authors acknowledge financial support from a National Health and Medical Research Council (NHMRC) project grant #511109. K. Barraclough is currently supported by a NHMRC medical/dental postgraduate scholarship. C. Staatz is currently supported by a Lions Medical Research Fellowship. The authors have no conflicts of interest to declare that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bergmann, T.K., Barraclough, K.A., Lee, K.J. et al. Clinical Pharmacokinetics and Pharmacodynamics of Prednisolone and Prednisone in Solid Organ Transplantation. Clin Pharmacokinet 51, 711–741 (2012). https://doi.org/10.1007/s40262-012-0007-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-012-0007-8