Abstract
Atomoxetine was first licensed to treat attention-deficit/hyperactivity disorder (ADHD) in children and adolescents in the US in 2002. The aim of this paper is to comprehensively review subsequent publications addressing the efficacy of atomoxetine in 6- to 18-year-olds with ADHD. We identified 125 eligible papers using a predefined search strategy. Overall, these papers demonstrate that atomoxetine is an effective treatment for the core ADHD symptoms (effect sizes 0.6–1.3, vs. placebo, at 6–18 weeks), and improves functional outcomes and quality of life, in various pediatric populations with ADHD (i.e., males/females, patients with co-morbidities, children/adolescents, and with/without prior exposure to other ADHD medications). Initial responses to atomoxetine may be apparent within 1 week of treatment, but can take longer (median 23 days in a 6-week study; n = 72). Responses often build gradually over time, and may not be robust until after 3 months. A pooled analysis of six randomized placebo-controlled trials (n = 618) indicated that responses at 4 weeks may predict response at 6–9 weeks, although another pooled analysis of open-label data (n = 338) suggests that the probability of a robust response to atomoxetine [≥40 % decrease in ADHD–Rating Scale (ADHD-RS) scores] may continue to increase beyond 6–9 weeks. Atomoxetine may demonstrate similar efficacy to methylphenidate, particularly immediate-release methylphenidate, although randomized controlled trials are generally limited by short durations (3–12 weeks). In conclusion, notwithstanding these positive findings, before initiating treatment with atomoxetine, it is important that the clinician sets appropriate expectations for the patient and their family with regard to the likelihood of a gradual response, which often builds over time.

Similar content being viewed by others
Notes
Response was defined as “at least a 25 % decrease from baseline on the ADHD-RS-IV [ADHD–Rating Scale–IV] total score”.
Remission was defined as “each ADHD-RS-IV item score ≤1 at the end of the treatment”.
However, these results should be viewed with caution, particularly as the congress abstract does not state a definition for “treatment failure”, and we are not aware of these results having been published in a full-length peer-reviewed study paper or of being replicated.
The primary outcome measure was comparison of mean time to relapse, defined as an increase in ADHD-RS total score to 90 % of the score at study entry and an increase in CGI–Severity [CGI-S] score of ≥2 points above the CGI-S score at the end of the initial 10 weeks of treatment. A secondary definition of relapse (≥50 % worsening in ADHD-RS severity score above the last pre-randomization visit) resulted in similar relapse rates [atomoxetine, 6/81 (7.4 %); placebo, 16/82 (19.5 %); p = 0.037; relative risk ratio, 3.0 (95 % CI 1.2–7.6)]. A greater number of patients who received atomoxetine completed the 6-month randomized phase [65/79 (82.3 %)] than did those who received placebo [54/81 (66.7 %); p = 0.030].
The definition of non-inferiority was “if the lower limit of the 2-sided 95 % CI for the difference in proportion of responders (ATX [atomoxetine] minus MPH [methylphenidate]) is greater than −15%, ATX will be considered non-inferior to MPH”.
In the meta-analysis paper [143], and the source study in this paper, it is unclear whether ‘deprenyl’ referred to L-deprenyl (also known as selegiline), D-deprenyl, or a racemic mixture.
References
Young ME, Galvan T, Reidy BL, et al. Family functioning deficits in bipolar disorder and ADHD in youth. J Affect Disord. 2013;150(3):1096–102.
Langley K, Fowler T, Ford T, et al. Adolescent clinical outcomes for young people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2010;196(3):235–40.
Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29(4):546–57.
Babinski DE, Pelham WE Jr, Molina BS, et al. Late adolescent and young adult outcomes of girls diagnosed with ADHD in childhood: an exploratory investigation. J Atten Disord. 2011;15(3):204–14.
Rasmussen P, Gillberg C. Natural outcome of ADHD with developmental coordination disorder at age 22 years: a controlled, longitudinal, community-based study. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1424–31.
Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. Med Gen Med. 2006;8(3):12.
Dalsgaard S, Mortensen PB, Frydenberg M, Thomsen PH. Long-term criminal outcome of children with attention deficit hyperactivity disorder. Crim Behav Ment Health. 2013;23(2):86–98.
Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990–1002.
Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2(2):104–13.
Willcutt EG. The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics. 2012;9(3):490–9.
Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942–8.
Polanczyk GV, Willcutt EG, Salum GA, et al. ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. Int J Epidemiol. 2014;43(2):434–42.
Kornfield R, Watson S, Higashi AS, et al. Effects of FDA advisories on the pharmacologic treatment of ADHD, 2004–2008. Psychiatr Serv. 2013;64(4):339–46.
Cardo E, Porsdal V, Quail D, et al. Fast vs. slow switching from stimulants to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2013;23(4):252–61.
Banaschewski T, Roessner V, Dittmann RW, et al. Non-stimulant medications in the treatment of ADHD. Eur Child Adolesc Psychiatry. 2004;13(Suppl 1):102–16.
Upadhyaya HP, Desaiah D, Schuh KJ, et al. A review of the abuse potential assessment of atomoxetine: a nonstimulant medication for attention-deficit/hyperactivity disorder. Psychopharmacology. 2013;226(2):189–200.
Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2002;63(12):1140–7.
Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics. 2001;108(5):E83.
Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry. 2002;159(11):1896–901.
Weiss M, Tannock R, Kratochvil C, et al. A randomized, placebo-controlled study of once-daily atomoxetine in the school setting in children with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44(7):647–55.
Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics. 2004;114(1):e1–8.
Bushe CJ, Savill NC. Systematic review of atomoxetine data in childhood and adolescent attention-deficit hyperactivity disorder 2009–2011: focus on clinical efficacy and safety. J Psychopharmacol. 2014;28(3):204–11.
Schwartz S, Correll CU. Efficacy and safety of atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: results from a comprehensive meta-analysis and metaregression. J Am Acad Child Adolesc Psychiatry. 2014;53(2):174–87.
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145–57.
Agster KL, Bates AT, Cain RE, et al. The role of cortical norepinephrine in the development of executive function. Neuropsychopharmacology. 2011;36:S83.
Gehlert DR, Schober DA, Hemrick-Luecke SK, et al. Novel halogenated analogs of tomoxetine that are potent and selective inhibitors of norepinephrine uptake in brain. Neurochem Int. 1995;26(1):47–52.
Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340(2–3):249–58.
Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther. 1982;222(1):61–5.
Bolden-Watson C, Richelson E. Blockade by newly developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci. 1993;52(12):1023–9.
Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology. 2002;27(5):699–711.
Volz TJ, Farnsworth SJ, Rowley SD, et al. Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and dopamine D2 receptors. J Pharmacol Exp Ther. 2008;327(1):161–7.
Calipari ES, Ferris MJ, Salahpour A, et al. Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun. 2013;4:2720.
Hutson PH, Pennick M, Secker R. Preclinical pharmacokinetics, pharmacology and toxicology of lisdexamfetamine: a novel d-amphetamine pro-drug. Neuropharmacology. 2014;87C:41–50.
Fan X, Hess EJ. D2-like dopamine receptors mediate the response to amphetamine in a mouse model of ADHD. Neurobiol Dis. 2007;26(1):201–11.
Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60(2):284–95.
Molina BS, Pelham WE Jr. Childhood predictors of adolescent substance use in a longitudinal study of children with ADHD. J Abnorm Psychol. 2003;112(3):497–507.
Upadhyaya HP, Rose K, Wang W, et al. Attention-deficit/hyperactivity disorder, medication treatment, and substance use patterns among adolescents and young adults. J Child Adolesc Psychopharmacol. 2005;15(5):799–809.
Galéra C, Melchior M, Chastang JF, et al. Childhood and adolescent hyperactivity-inattention symptoms and academic achievement 8 years later: the GAZEL Youth study. Psychol Med. 2009;39(11):1895–906.
Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2010;33(2):357–73.
Tanaka Y, Rohde LA, Jin L, et al. A meta-analysis of the consistency of atomoxetine treatment effects in pediatric patients with attention-deficit/hyperactivity disorder from 15 clinical trials across four geographic regions. J Child Adolesc Psychopharmacol. 2013;23(4):262–70.
Rommelse NN, Geurts HM, Franke B, et al. A review on cognitive and brain endophenotypes that may be common in autism spectrum disorder and attention-deficit/hyperactivity disorder and facilitate the search for pleiotropic genes. Neurosci Biobehav Rev. 2011;35(6):1363–96.
Rommelse NN, Franke B, Geurts HM, et al. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19(3):281–95.
Udvardi PT, Föhr KJ, Henes C, et al. Atomoxetine affects transcription/translation of the NMDA receptor and the norepinephrine transporter in the rat brain—an in vivo study. Drug Des Devel Ther. 2013;7:1433–46.
STRATTERA®. Clinical study results. Eli Lilly and Co. http://www.lillytrials.com/results/Strattera.pdf. Accessed 5 Dec 2014.
STRATTERA®. Summary of product characteristics. Eli Lilly and Co. http://www.medicines.org.uk/emc/medicine/14482. Accessed 27 Nov 2014.
STRATTERA®. Full prescribing information. Eli Lilly and Co. http://www.pi.lilly.com/us/strattera-pi.pdf. Accessed 27 Nov 2014.
Thurstone C, Riggs PD, Salomonsen-Sautel S, Mikulich-Gilbertson SK. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(6):573–82.
Schulz KP, Fan J, Bédard AC, et al. Common and unique therapeutic mechanisms of stimulant and nonstimulant treatments for attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2012;69(9):952–61.
Ramoz N, Boni C, Downing AM, et al. A haplotype of the norepinephrine transporter (Net) gene Slc6a2 is associated with clinical response to atomoxetine in attention-deficit hyperactivity disorder (ADHD). Neuropsychopharmacology. 2009;34(9):2135–42.
Yang L, Qian Q, Liu L, et al. Adrenergic neurotransmitter system transporter and receptor genes associated with atomoxetine response in attention-deficit hyperactivity disorder children. J Neural Transm. 2013;120(7):1127–33.
Michelson D, Read HA, Ruff DD, et al. CYP2D6 and clinical response to atomoxetine in children and adolescents with ADHD. J Am Acad Child Adolesc Psychiatry. 2007;46(2):242–51.
Hazell P, Becker K, Nikkanen EA, et al. Relationship between atomoxetine plasma concentration, treatment response and tolerability in attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Atten Defic Hyperact Disord. 2009;1(2):201–10.
Newcorn JH, Sutton VK, Weiss MD, Sumner CR. Clinical responses to atomoxetine in attention-deficit/hyperactivity disorder: the Integrated Data Exploratory Analysis (IDEA) study. J Am Acad Child Adolesc Psychiatry. 2009;48(5):511–8.
Dickson RA, Maki E, Gibbins C, et al. Time courses of improvement and symptom remission in children treated with atomoxetine for attention-deficit/hyperactivity disorder: analysis of Canadian open-label studies. Child Adolesc Psychiatry Ment Health. 2011;5(14).
Wilens TE, Newcorn JH, Kratochvil CJ, et al. Long-term atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Pediatr. 2006;149(1):112–9.
Buitelaar JK, Michelson D, Danckaerts M, et al. A randomized, double-blind study of continuation treatment for attention-deficit/hyperactivity disorder after 1 year. Biol Psychiatry. 2007;61(5):694–9.
Perwien AR, Faries DE, Kratochvil CJ, et al. Improvement in health-related quality of life in children with ADHD: an analysis of placebo controlled studies of atomoxetine. J Dev Behav Pediatr. 2004;25(4):264–71.
Urion DK. Atomoxetine “treatment failures” usually respond to dosing changes. Ann Neurol. 2012;72:S186–7.
Block SL, Kelsey D, Coury D, et al. Once-daily atomoxetine for treating pediatric attention-deficit/hyperactivity disorder: comparison of morning and evening dosing. Clin Pediatr. 2009;48(7):723–33.
Dittmann RW, Schacht A, Helsberg K, et al. Atomoxetine versus placebo in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a double-blind, randomized, multicenter trial in Germany. J Child Adolesc Psychopharmacol. 2011;21(2):97–110.
Kratochvil CJ, Bohac D, Harrington M, et al. An open-label trial of tomoxetine in pediatric attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11(2):167–70.
Cho S, Lee SI, Yoo H, et al. A randomized, open-label assessment of response to various doses of atomoxetine in Korean pediatric outpatients with attention-deficit/hyperactivity disorder. Psychiatry Investig. 2011;8(2):141–8.
Takahashi M, Takita Y, Yamazaki K, et al. A randomized, double-blind, placebo-controlled study of atomoxetine in Japanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(4):341–50.
Wietecha LA, Williams DW, Herbert M, et al. Atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(6):719–30.
Newcorn JH, Michelson D, Kratochvil CJ, et al. Low-dose atomoxetine for maintenance treatment of attention-deficit/hyperactivity disorder. Pediatrics. 2006;118(6):e1701–6.
Spencer T, Biederman J, Heiligenstein J, et al. An open-label, dose-ranging study of atomoxetine in children with attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11(3):251–65.
Kratochvil CJ, Michelson D, Newcorn JH, et al. High-dose atomoxetine treatment of ADHD in youths with limited response to standard doses. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1128–37.
Dittmann RW, Cardo E, Nagy P, et al. Efficacy and safety of lisdexamfetamine dimesylate and atomoxetine in the treatment of attention-deficit/hyperactivity disorder: a head-to-head, randomized, double-blind, phase IIIb study. CNS Drugs. 2013;27(12):1081–92.
Martenyi F, Zavadenko NN, Jarkova NB, et al. Atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder: a 6-week, randomized, placebo-controlled, double-blind trial in Russia. Eur Child Adolesc Psychiatry. 2010;19(1):57–66.
Biederman J, Heiligenstein JH, Faries DE, et al. Efficacy of atomoxetine versus placebo in school-age girls with attention-deficit/hyperactivity disorder. Pediatrics. 2002;110(6):e75.
Dittmann RW, Wehmeier PM, Schacht A, et al. Atomoxetine treatment and ADHD-related difficulties as assessed by adolescent patients, their parents and physicians. Child Adolesc Psychiatry Ment Health. 2009;3(1):21.
Buitelaar JK, Danckaerts M, Gillberg C, et al. A prospective, multicenter, open-label assessment of atomoxetine in non-North American children and adolescents with ADHD. Eur Child Adolesc Psychiatry. 2004;13(4):249–57.
Tamayo JM, Pumariega A, Rothe EM, et al. Latino versus Caucasian response to atomoxetine in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18(1):44–53.
Durell TM, Pumariega AJ, Rothe EM, et al. Effects of open-label atomoxetine on African-American and Caucasian pediatric outpatients with attention-deficit/hyperactivity disorder. Ann Clin Psychiatry. 2009;21(1):26–37.
Block SL, Williams D, Donnelly CL, et al. Post hoc analysis: early changes in ADHD-RS items predict longer term response to atomoxetine in pediatric patients. Clin Pediatr. 2010;49(8):768–76.
Montoya A, Quail D, Anand E, et al. Prognostic factors of improvement in health related quality of life in children and adolescents with attention deficit/hyperactivity disorder, after atomoxetine treatment. Eur Psychiatry. 2012;27(Suppl 1):1.
Michelson D, Buitelaar JK, Danckaerts M, et al. Relapse prevention in pediatric patients with ADHD treated with atomoxetine: a randomized, double-blind, placebo-controlled study. J Am Acad Child Adolesc Psychiatry. 2004;43(7):896–904.
Hazell P, Zhang S, Wolańczyk T, et al. Comorbid oppositional defiant disorder and the risk of relapse during 9 months of atomoxetine treatment for attention-deficit/hyperactivity disorder. Eur Child Adolesc Psychiatry. 2006;15(2):105–10.
Kratochvil CJ, Wilens TE, Greenhill LL, et al. Effects of long-term atomoxetine treatment for young children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45(8):919–27.
Wehmeier PM, Dittmann RW, Schacht A, et al. Morning and evening behavior in children and adolescents treated with atomoxetine once daily for attention-deficit/hyperactivity disorder (ADHD): findings from two 24-week, open-label studies. Child Adolesc Psychiatry Ment Health. 2009;3(1):5.
Wehmeier PM, Schacht A, Ulberstad F, et al. Does atomoxetine improve executive function, inhibitory control, and hyperactivity? Results from a placebo-controlled trial using quantitative measurement technology. J Clin Psychopharmacol. 2012;32(5):653–60.
Wilens TE, Hammerness P, Utzinger L, et al. An open study of adjunct OROS-methylphenidate in children and adolescents who are atomoxetine partial responders: I. Effectiveness. J Child Adolesc Psychopharmacol. 2009;19(5):485–92.
Wigal SB, McGough JJ, McCracken JT, et al. A laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with attention deficit/hyperactivity disorder. J Atten Disord. 2005;9(1):275–89.
Faraone SV, Wigal SB, Hodgkins P. Forecasting three-month outcomes in a laboratory school comparison of mixed amphetamine salts extended release (Adderall XR) and atomoxetine (Strattera) in school-aged children with ADHD. J Atten Disord. 2007;11(1):74–82.
Roskell NS, Setyawan J, Zimovetz EA, Hodgkins P. Systematic review and network meta-analysis of treatments used for attention deficit hyperactivity disorder (ADHD). Value Health. 2012;15(7):A334.
Sikirica V, Findling RL, Signorovitch J, et al. Comparative efficacy of guanfacine extended release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: applying matching-adjusted indirect comparison methodology. CNS Drugs. 2013;27(11):943–53.
Bastiaens L. Effectiveness and tolerability of atomoxetine in a real-world ADHD population: nonrandomized comparison with stimulants. Psychiatry. 2007;4(12):44–8.
Whalen CK, Henker B, Ishikawa SS, et al. Atomoxetine versus stimulants in the community treatment of children with ADHD: an electronic diary study. J Atten Disord. 2010;13(4):391–400.
Fuentes J, Danckaerts M, Cardo E, et al. Long-term quality-of-life and functioning comparison of atomoxetine versus other standard treatment in pediatric attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2013;33(6):766–74.
Hazell PL, Kohn MR, Dickson R, et al. Core ADHD symptom improvement with atomoxetine versus methylphenidate: a direct comparison meta-analysis. J Atten Disord. 2011;15(8):674–83.
Hanwella R, Senanayake M, de Silva V. Comparative efficacy and acceptability of methylphenidate and atomoxetine in treatment of attention deficit hyperactivity disorder in children and adolescents: a meta-analysis. BMC Psychiatry. 2011;11:176.
Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: a prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry. 2002;41(7):776–84.
Sangal RB, Owens J, Allen AJ, et al. Effects of atomoxetine and methylphenidate on sleep in children with ADHD. Sleep. 2006;29(12):1573–85.
Prasad S, Harpin V, Poole L, et al. A multi-centre, randomised, open-label study of atomoxetine compared with standard current therapy in UK children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Curr Med Res Opin. 2007;23(2):379–94.
Wang Y, Zheng Y, Du Y, et al. Atomoxetine versus methylphenidate in paediatric outpatients with attention deficit hyperactivity disorder: a randomized, double-blind comparison trial. Aust NZ J Psychiatry. 2007;41(3):222–30.
Newcorn JH, Kratochvil CJ, Allen AJ, et al. Atomoxetine and osmotically released methylphenidate for the treatment of attention deficit hyperactivity disorder: acute comparison and differential response. Am J Psychiatry. 2008;165(6):721–30.
Kemner JE, Starr HL, Ciccone PE, et al. Outcomes of OROS methylphenidate compared with atomoxetine in children with ADHD: a multicenter, randomized prospective study. Adv Ther. 2005;22(5):498–512.
Yildiz O, Sismanlar SG, Memik NC, et al. Atomoxetine and methylphenidate treatment in children with ADHD: the efficacy, tolerability and effects on executive functions. Child Psychiatry Hum Dev. 2011;42(3):257–69.
Starr HL, Kemner J. Multicenter, randomized, open-label study of OROS methylphenidate versus atomoxetine: treatment outcomes in African-American children with ADHD. J Natl Med Assoc. 2005;97(10):11S–6S.
Mazzone L, Postorino V, Reale L, et al. Self-esteem evaluation in children and adolescents suffering from ADHD. Clin Pract Epidemiol Ment Health. 2013;9:96–102.
Biederman J, Spencer TJ, Newcorn JH, et al. Effect of comorbid symptoms of oppositional defiant disorder on responses to atomoxetine in children with ADHD: a meta-analysis of controlled clinical trial data. Psychopharmacology. 2007;190(1):31–41.
Dell’Agnello G, Maschietto D, Bravaccio C, et al. Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled Italian study. Eur Neuropsychopharmacol. 2009;19(11):822–34.
Brown RT, Perwien A, Faries DE, et al. Atomoxetine in the management of children with ADHD: effects on quality of life and school functioning. Clin Pediatr. 2006;45(9):819–27.
Cheng JY, Chen RY, Ko JSN, et al. Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents—meta-analysis and meta-regression analysis. Psychopharmacology. 2007;194(2):197–209.
Dittmann RW, Wehmeier PM, Schacht A, et al. Self-esteem in adolescent patients with attention-deficit/hyperactivity disorder during open-label atomoxetine treatment: psychometric evaluation of the Rosenberg Self-Esteem Scale and clinical findings. Atten Defic Hyperact Disord. 2009;1(2):187–200.
Escobar R, Montoya A, Polavieja P, et al. Evaluation of patients’ and parents’ quality of life in a randomized placebo-controlled atomoxetine study in attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19(3):253–63.
Escobar R, Schacht A, Wehmeier PM, Wagner T. Quality of life and attention-deficit/hyperactivity disorder core symptoms: a pooled analysis of 5 non-US atomoxetine clinical trials. J Clin Psychopharmacol. 2010;30(2):145–51.
Donnelly C, Faries D, Swensen A, et al. The effect of atomoxetine on the social and family functioning of children and adolescents with attention-deficit/hyperactivity disorder (ADHD). Eur Neuropsychopharmacol. 2002;12(Suppl 3):S437.
Maziade M, Rouleau N, Lee B, et al. Atomoxetine and neuropsychological function in children with attention-deficit/hyperactivity disorder: results of a pilot study. J Child Adolesc Psychopharmacol. 2009;19(6):709–18.
Van Der Kolk A, Bouwmans C, Schawo S, et al. The quality of life of children with ADHD and their parents: results of EQ-5D and Kidscreen-10. Value Health. 2011;14(7):A294–5.
Perwien AR, Kratochvil CJ, Faries DE, et al. Atomoxetine treatment in children and adolescents with attention-deficit hyperactivity disorder: what are the long-term health-related quality-of-life outcomes? J Child Adolesc Psychopharmacol. 2006;16(6):713–24.
Matza LS, Rentz AM, Secnik K, et al. The link between health-related quality of life and clinical symptoms among children with attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2004;25(3):166–74.
Bastiaens L. Improvement in global psychopathology increases quality of life during treatment of ADHD with atomoxetine or stimulants. Psychiatr Q. 2011;82(4):303–8.
Buitelaar JK, Wilens TE, Zhang S, et al. Comparison of symptomatic versus functional changes in children and adolescents with ADHD during randomized, double-blind treatment with psychostimulants, atomoxetine, or placebo. J Child Psychol Psychiatry. 2009;50(3):335–42.
Waxmonsky JG, Waschbusch DA, Pelham WE, et al. Effects of atomoxetine with and without behavior therapy on the school and home functioning of children with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2010;71(11):1535–51.
Wehmeier PM, Dittmann RW, Schacht A, et al. Effectiveness of atomoxetine and quality of life in children with attention-deficit/hyperactivity disorder as perceived by patients, parents, and physicians in an open-label study. J Child Adolesc Psychopharmacol. 2007;17(6):813–30.
Wehmeier PM, Schacht A, Dittmann RW, et al. Effect of atomoxetine on quality of life and family burden: results from a randomized, placebo-controlled, double-blind study in children and adolescents with ADHD and comorbid oppositional defiant or conduct disorder. Qual Life Res. 2011;20(5):691–702.
Newcorn JH, Spencer TJ, Biederman J, et al. Atomoxetine treatment in children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2005;44(3):240–8.
Dumitru I, Salan A. Treating children with epilepsy and comorbid attention-deficit/hyperactivity disorder (ADHD). Eur J Neurol. 2012;19:208.
Shang CY, Gau SS. Improving visual memory, attention, and school function with atomoxetine in boys with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(5):353–63.
Svanborg P, Thernlund G, Gustafsson PA, et al. Atomoxetine improves patient and family coping in attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in Swedish children and adolescents. Eur Child Adolesc Psychiatry. 2009;18(12):725–35.
Wehmeier PM, Schacht A, Dittmann RW, Banaschewski T. Minor differences in ADHD-related difficulties between boys and girls treated with atomoxetine for attention-deficit/hyperactivity disorder. Atten Defic Hyperact Disord. 2010;2(2):73–85.
Wehmeier PM, Schacht A, Escobar R, et al. Health-related quality of life in ADHD: a pooled analysis of gender differences in five atomoxetine trials. Atten Defic Hyperact Disord. 2012;4(1):25–35.
Wehmeier PM, Schacht A, Escobar R, et al. Differences between children and adolescents in treatment response to atomoxetine and the correlation between health-related quality of life and attention deficit/hyperactivity disorder core symptoms: meta-analysis of five atomoxetine trials. Child Adolesc Psychiatry Ment Health. 2010;4:30.
Saylor K, Williams DW, Schuh KJ, et al. Effects of atomoxetine on self-reported high-risk behaviors and health-related quality of life in adolescents with ADHD. Curr Med Res Opin. 2010;26(9):2087–95.
Quintana H, Cherlin EA, Duesenberg DA, et al. Transition from methylphenidate or amphetamine to atomoxetine in children and adolescents with attention-deficit/hyperactivity disorder—a preliminary tolerability and efficacy study. Clin Ther. 2007;29(6):1168–77.
Heiligenstein JH, Dunn DW, Busner J, et al. Efficacy of tomoxetine versus placebo in school-age children with ADHD who failed psychostimulant treatment. 154th Annual Meeting of the American Psychiatric Association. 2001 5–10 May; New Orleans, LA.
Hammerness P, Doyle R, Kotarski M, et al. Atomoxetine in children with attention-deficit hyperactivity disorder with prior stimulant therapy: a prospective open-label study. Eur Child Adolesc Psychiatry. 2009;18(8):493–8.
Bakken RJ, Paczkowski M, Kramer HP, et al. Effects of atomoxetine on attention-deficit/hyperactivity disorder in clinical pediatric treatment settings: a naturalistic study. Curr Med Res Opin. 2008;24(2):449–60.
Niederkirchner K, Slawik L, Wermelskirchen D, et al. Transitioning to OROS® methylphenidate from atomoxetine is effective in children and adolescents with ADHD. Expert Rev Neurother. 2011;11(4):499–508.
Montoya A, Hervas A, Cardo E, et al. Evaluation of atomoxetine for first-line treatment of newly diagnosed, treatment-naïve children and adolescents with attention deficit/hyperactivity disorder. Curr Med Res Opin. 2009;25(11):2745–54.
Mendez L, Singh P, Harrison G, et al. Academic outcomes in Asian children aged 8–11 years with attention-deficit/hyperactivity disorder treated with atomoxetine hydrochloride. Int J Psychiatry Clin Pract. 2011;15(2):145–56.
Wehmeier PM, Dittmann RW, Banaschewski T, Schacht A. Does stimulant pretreatment modify atomoxetine effects on core symptoms of ADHD in children assessed by quantitative measurement technology? J Atten Disord. 2014;18(2):105–16.
Svanborg P, Thernlund G, Gustafsson PA, et al. Efficacy and safety of atomoxetine as add-on to psychoeducation in the treatment of attention deficit/hyperactivity disorder: a randomized, double-blind, placebo-controlled study in stimulant-naïve Swedish children and adolescents. Eur Child Adolesc Psychiatry. 2009;18(4):240–9.
Wilens TE, Kratochvil C, Newcorn JH, Gao H. Do children and adolescents with ADHD respond differently to atomoxetine? J Am Acad Child Adolesc Psychiatry. 2006;45(2):149–57.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV). Washington, DC: American Psychiatric Association; 1994.
Bangs ME, Emslie GJ, Spencer TJ, et al. Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J Child Adolesc Psychopharmacol. 2007;17(4):407–20.
Zeiner P, Gjevik E, Weidle B. Response to atomoxetine in boys with high-functioning autism spectrum disorders and attention deficit/hyperactivity disorder. Acta Paediatr. 2011;100(9):1258–61.
Biederman J, Wigal SB, Spencer TJ, et al. A post hoc subgroup analysis of an 18-day randomized controlled trial comparing the tolerability and efficacy of mixed amphetamine salts extended release and atomoxetine in school-age girls with attention-deficit/hyperactivity disorder. Clin Ther. 2006;28(2):280–93.
Spencer TJ, Sallee FR, Gilbert DL, et al. Atomoxetine treatment of ADHD in children with comorbid Tourette syndrome. J Atten Disord. 2008;11(4):470–81.
Gilbert DL, Zhang J, Lipps TD, et al. Atomoxetine treatment of ADHD in Tourette syndrome: reduction in motor cortex inhibition correlates with clinical improvement. Clin Neurophysiol. 2007;118(8):1835–41.
Allen AJ, Kurlan RM, Gilbert DL, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology. 2005;65(12):1941–9.
Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF. Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry. 2009;48(9):884–93.
Chang K, Nayar D, Howe M, Rana M. Atomoxetine as an adjunct therapy in the treatment of co-morbid attention-deficit/hyperactivity disorder in children and adolescents with bipolar I or II disorder. J Child Adolesc Psychopharmacol. 2009;19(5):547–51.
Geller D, Donnelly C, Lopez F, et al. Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(9):1119–27.
Kratochvil CJ, Newcorn JH, Arnold LE, et al. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. J Am Acad Child Adolesc Psychiatry. 2005;44(9):915–24.
Bangs ME, Hazell P, Danckaerts M, et al. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder and oppositional defiant disorder. Pediatrics. 2008;121(2):e314–20.
Kaplan S, Heiligenstein J, West S, et al. Efficacy and safety of atomoxetine in childhood attention-deficit/hyperactivity disorder with comorbid oppositional defiant disorder. J Atten Disord. 2004;8(2):45–52.
Ercan ES, Akyol Ardic U, Kabukcu Basay B, et al. Atomoxetine response in the inattentive and combined subtypes of attention deficit hyperactivity disorder: a retrospective chart review. Atten Defic Hyperact Disord. 2013;5(4):377–85.
Wehmeier PM, Kipp L, Banaschewski T, et al. Does comorbid disruptive behavior modify the effects of atomoxetine on ADHD symptoms as measured by a continuous performance test and a motion tracking device? J Atten Disord. doi:10.1177/1087054712456739 (Epub 2012 Aug 28).
Waxmonsky JG, Waschbusch DA, Akinnusi O, Pelham WE. A comparison of atomoxetine administered as once versus twice daily dosing on the school and home functioning of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21(1):21–32.
van Wyk GW, Hazell PL, Kohn MR, et al. How oppositionality, inattention, and hyperactivity affect response to atomoxetine versus methylphenidate: a pooled meta-analysis. J Atten Disord. 2012;16(4):314–24.
Signorovitch J, Erder MH, Xie J, et al. Comparative effectiveness research using matching-adjusted indirect comparison: an application to treatment with guanfacine extended release or atomoxetine in children with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):130–7.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). Washington, DC: American Psychiatric Association; 2013.
Harfterkamp M, van de Loo-Neus G, Minderaa RB, et al. A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2012;51(7):733–41.
Harfterkamp M, Buitelaar JK, Minderaa RB, et al. Long-term treatment with atomoxetine for attention-deficit/hyperactivity disorder symptoms in children and adolescents with autism spectrum disorder: an open-label extension study. J Child Adolesc Psychopharmacol. 2013;23(3):194–9.
Charnsil C. Efficacy of atomoxetine in children with severe autistic disorders and symptoms of ADHD: an open-label study. J Atten Disord. 2011;15(8):684–9.
Posey DJ, Wiegand RE, Wilkerson J, et al. Open-label atomoxetine for attention-deficit/hyperactivity disorder symptoms associated with high-functioning pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2006;16(5):599–610.
Troost PW, Steenhuis MP, Tuynman-Qua HG, et al. Atomoxetine for attention-deficit/hyperactivity disorder symptoms in children with pervasive developmental disorders: a pilot study. J Child Adolescent Psychopharmacol. 2006;16(5):611–9.
Arnold LE, Aman MG, Cook AM, et al. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1196–205.
Mazzone L, Reale L, Mannino V, et al. Lower IQ is associated with decreased clinical response to atomoxetine in children and adolescents with attention-deficit hyperactivity disorder. CNS Drugs. 2011;25(6):503–9.
Fernández-Jaén A, Fernández-Mayoralas DM, Calleja Pérez B, et al. Atomoxetine for attention deficit hyperactivity disorder in mental retardation. Pediatr Neurol. 2010;43(5):341–7.
Shaywitz SE, Shaywitz BA, Wietecha LA, et al. Effects of atomoxetine on reading abilities in children with dyslexia and children with attention deficit/hyperactivity disorder and comorbid dyslexia. Ann Neurol. 2012;72:S204.
Sumner CR, Gathercole S, Greenbaum M, et al. Atomoxetine for the treatment of attention-deficit/hyperactivity disorder (ADHD) in children with ADHD and dyslexia. Child Adolesc Psychiatry Ment Health. 2009;3:40.
Gau SSF, Huang YS, Soong WT, et al. A randomized, double-blind, placebo-controlled clinical trial on once-daily atomoxetine hydrochloride in Taiwanese children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17(4):447–60.
Biederman J, Gao H, Rogers AK, Spencer TJ. Comparison of parent and teacher reports of attention-deficit/hyperactivity disorder symptoms from two placebo-controlled studies of atomoxetine in children. Biol Psychiatry. 2006;60(10):1106–10.
Bohnstedt BN, Kronenberger WG, Dunn DW, et al. Investigator ratings of ADHD symptoms during a randomized, placebo-controlled trial of atomoxetine: a comparison of parents and teachers as informants. J Atten Disord. 2005;8(4):153–9.
Vakula N, Vasyanina Y, Gorbunova Z, et al. Efficacy of Strattera in children and adolescents with attention deficit hyperactivity disorder. Neurosci Behav Physiol. 2010;40(9):1034–7.
Sumner CR, Haynes VS, Teicher MH, Newcorn JH. Does placebo response differ between objective and subjective measures in children with attention-deficit/hyperactivity disorder? Postgrad Med. 2010;122(5):52–61.
Kratochvil CJ, Milton DR, Vaughan BS, Greenhill LL. Acute atomoxetine treatment of younger and older children with ADHD: a meta-analysis of tolerability and efficacy. Child Adolesc Psychiatry Ment Health. 2008;2(1):25.
Acknowledgments and conflicts of interest
N.C. Savill, K. A. Day, E. Anand, T. Treuer, and H. P. Upadhyaya are full-time employees and stock holders of Eli Lilly and Co.
J. K. Buitelaar has been a consultant, a member of an advisory board, and/or a speaker for Janssen-Cilag BV, Eli Lilly, Bristol-Myers Squibb, Organon/Schering Plough, UCB, Shire, Medice, and Servier.
D. Coghill has served in an advisory/consultancy role for Flynn Pharma, Otsuka, Lilly, Janssen, Medice, Pfizer, Schering-Plough, Shire, and Vifor. He received conference attendance support, conference support, or speaker’s fees from Flynn Pharma, Eli Lilly, Janssen, Medice, Novartis, and Shire. He has been involved in clinical trials conducted by Eli Lilly and Shire and has received research funding from Eli Lilly, Janssen, Shire, and Vifor.
This review was funded by Eli Lilly and Company. K. A. Day performed the predefined literature database searches, and J. K. Buitelaar and N. C. Savill selected relevant literature from the search results. All authors contributed to the conception/design of the manuscript, and critically reviewed and thus participated in writing the manuscript. All authors approved the final version of the manuscript, and agree to be accountable for this work. Michael Riley, PhD, from Trilogy Writing and Consulting GmbH, Frankfurt, Germany, provided medical writing support on behalf of Eli Lilly and Company.
Author information
Authors and Affiliations
Corresponding author
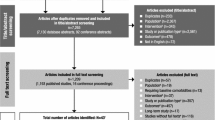
Appendix
Appendix
1.1 Strategy used in ‘Search 1’
Database: EMBASE <1974 to 2013 July 25>, Ovid MEDLINE® <1946 to July Week 3 2013>, Ovid MEDLINE® In-Process and Other Non-Indexed Citations <July 25, 2013>
1 exp atomoxetine/(3060)
2 exp attention deficit disorder/(54376)
3 1 and 2 (2079)
4 limit 3 to [human and (child <unspecified age> or preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>)] [Limit not valid in Ovid MEDLINE®, Ovid MEDLINE® In-Process; records were retained] (725)
5 limit 4 to year = “2001–Current” (722)
6 5 use oemezd (722)
7 atomoxetine.mp. (4248)
8 tomoxetine.mp. (146)
9 LY139603.mp. (17)
10 7 or 8 or 9 (4289)
11 exp Attention Deficit Disorder with Hyperactivity/(54376)
12 10 and 11 (2818)
13 limit 12 to [humans and year = “2001–Current” and “all child (0 to 18 years)”] [Limit not valid in EMBASE; records were retained] (2468)
14 13 use mesz, prem (476)
15 6 or 14 (1198)
16 remove duplicates from 15 (879)
Rights and permissions
About this article
Cite this article
Savill, N.C., Buitelaar, J.K., Anand, E. et al. The Efficacy of Atomoxetine for the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Comprehensive Review of Over a Decade of Clinical Research. CNS Drugs 29, 131–151 (2015). https://doi.org/10.1007/s40263-014-0224-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40263-014-0224-9