Abstract
Purpose. The purpose of the present study is to investigate the expression of canalicular multispecific organic anion transporter (cMOAT) by its cDNA transfection in polarized Madin-Darby canine kidney cells (MDCK).
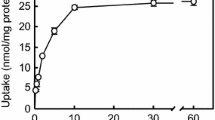
Methods. MDCK cells were transfected with an expression vector (pCXN2) containing the rat cMOAT cDNA with lipofectamine to obtain the stable transfectant under G418. Cells from a single colony whose cMOAT expression was the highest were seeded to form a tight epithelial monolayer on microporous membrane filters. Export of glutathione S-bimane (GS-B) from monolayers was determined after preloading its precursor, monochloro bimane (MCB).
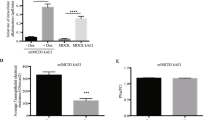
Results. A comparable amount of GS-B was excreted to the apical and basal compartments in the vector-transfected cells. In contrast, in cMOAT-transfected cells, the amount apically excreted was approximately twice that excreted into the basal compartment. Cyclosporin A (CsA) (30μM), an inhibitor of cMOAT at higher concentrations, inhibited the preferential apical export of GS-B from cMOAT-transfected cells.
Conclusions. Rat cMOAT is functionally expressed on the apical membrane of MDCK cells after transfection.
Similar content being viewed by others
REFERENCES
M. Yamazaki, H. Suzuki, and Y. Sugiyama. Recent advances in carrier-mediated hepatic uptake and biliary excretion of xenobiotics. Pharm. Res. 13:497–513 (1996).
R. P. Oude Elferink, D. K. Meijer, F. Kuipers, P. L. Jansen, A. K. Groen, and G. M. Groothuis. Hepatobiliary secretion of organic compounds; molecular mechanisms of membrane transport. Biochim. Biophys. Acta 1241:215–268 (1995).
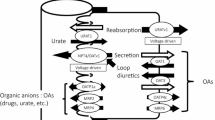
D. Keppler and J. König. Hepatic canalicular membrane 5: Expression and localization of the conjugate export pump encoded by the MRP2 (cMRP/cMOAT) gene in liver. FASEB J. 11:509–16 (1997).
M. Müller, E. G. de Vries, and P. L. Jansen. role of multidrug resistance protein (MRP) in glutathione S-conjugate transport in mammalian cells. J. Hepatol. 24:100–108 (1996).
X. Y. Chu, Y. Kato, K. Ni'inuma, K. I. Sudo, H. Hakusui, and Y. Sugiyama. Multispecific organic anion transporter is responsible for the biliary excretion of the camptothecin derivative irinotecan and its metabolites in rats. J. Pharmacol. Exp. Ther. 281:304–314 (1997).
X. Y. Chu, Y. Kato, and Y. Sugiyama. Multiplicity of biliary excretion mechanisms for irinotecan, CPT-11, and its metabolites in rats. Cancer Res. 57:1934–1938 (1997).
M. Masuda, Y. I'Izuka, M. Yamazaki, R. Nishigaki, Y. Kato, K. Ni'inuma, H. Suzuki, and Y. Sugiyama. Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Res. 57:3506–3510 (1997).
H. Ishizuka, K. Konno, H. Naganuma, K. Sasahara, Y. Kawahara, K. Ni'inuma, H. Suzuki, and Y. Sugiyama. Temocaprilat, a novel angiotensin-converting enzyme inhibitor, is excreted in bile via an ATP-dependent active transporter (cMOAT) that is deficient in Eisai hyperbilirubinemic mutant rats (EHBR). J. Pharmacol. Exp. Ther. 280:1304–1311 (1997).
C. C. Paulusma, P. J. Bosma, G. J. Zaman, C. T. Bakker, M. Otter, G. L. Scheffer, R. J. Scheper, P. Borst, and R. P. Oude Elferink. Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128 (1996).
M. Büchler, J. König, M. Brom, J. Kartenbeck, H. Spring, T. Horie, and D. Keppler. cDNA cloning of the hepatocyte canalicular isoform of the multidrug resistance protein, cMrp, reveals a novel conjugate export pump deficient in hyperbilirubinemic mutant rats. J. Biol. Chem. 271:15091–15098 (1996).
K. Taniguchi, M. Wada, K. Kohno, T. Nakamura, T. Kawabe, M. Kawakami, K. Kagotani, K. Okumura, S. Akiyama, and M. Kuwano. A human canalicular multispecific organic anion transporter (cMOAT) gene is overexpressed in cisplatin-resistant human cancer cell lines with decreased drug accumulation. Cancer Res. 56:4124–4129 (1996).
K. Ito, H. Suzuki, T. Hirohashi, K. Kume, T. Shimizu, and Y. Sugiyama. Molecular cloning of canalicular multispecific organic anion transporter defective in EHBR. Am. J. Physiol. 272:G16–22 (1997).
J. Madon, U. Eckhardt, T. Gerloff, B. Stieger, and P. J. Meier. Functional expression of the rat liver canalicular isoform of the multidrug resistance-associated protein. FEBS Lett. 406:75–78 (1997).
C. C. Paulusma, M. Kool, P. J. Bosma, G. L. Scheffer, F. ter Borg, R. J. Scheper, G. N. Tytgat, P. Borst, F. Baas, and R. P. Oude Elferink. A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology 25:1539–1542 (1997).
J. Kartenbeck, U. Leuschner, R. Mayer, and D. Keppler. Absence of the canalicular isoform of the MRP gene-encoded conjugate export pump from the hepatocytes in Dubin-Johnson syndrome. Hepatology 23:1061–1066 (1996).
M. Wada, S. Toh, K. Taniguchi, T. Nakamura, T. Uchiumi, K. Kohno, I. Yoshida, A. Kimura, S. Sakisaka, Y. Adachi, and M. Kuwano. Mutations in the canilicular multispecific organic anion transporter (cMOAT) gene, a novel ABC transporter, in patients with hyperbilirubinemia II/Dubin-Johnson syndrome. Hum. Mol. Genet. 7:203–7 (1998).
K. Ito, H. Suzuki, T. Hirohashi, K. Kume, T. Shimizu, and Y. Sugiyama. Functional analysis of a canalicular multispecific organic anion transporter cloned from rat liver. J. Biol. Chem. 273:1684–1688 (1998).
K. Koike, T. Kawabe, T. Tanaka, S. Toh, T. Uchiumi, M. Wada, S. Akiyama, M. Ono, and M. Kuwano. A canalicular multispecific organic anion transporter (cMOAT) antisense cDNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res. 57:5475–9 (1997).
R. Evers, G. J. Zaman, L. van Deemter, H. Jansen, J. Calafat, L. C. Oomen, R. P. Oude Elferink, P. Borst, and A. H. Schinkel. Basolateral localization and export activity of the human multidrug resistance-associated protein in polarized pig kidney cells. J. Clin. Invest. 97:1211–1218 (1996).
R. P. Oude Elferink, C. T. Bakker, H. Roelofsen, E. Middelkoop, R. Ottenhoff, M. Heijn, and P. L. Jansen. Accumulation of organic anion in intracellular vesicles of cultured rat hepatocytes is mediated by the canalicular multispecific organic anion transporter. Hepatology 17:434–444 (1993).
H. Niwa, K. Yamamura, and J. Miyazaki. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193–199 (1991).
K. Ito, H. Suzuki, T. Hirohashi, K. Kazuhiko, T. Shimizu, and Y. Sugiyama. Expression of the putative ATP-binding cassette region, homologous to that in multidrug resistance associated protein (MRP), is hereditarily defective in Eisai hyperbirilubinemic rats (EHBR). Int. Hepatol. Commun. 4:292–299 (1996).
O. H. Lowry, N. J. Roserough, A. L. Farr, and R. J. Randall. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275 (1951).
M. Böhme, M. Büchler, M. Müller, and D. Keppler. Differential inhibition by cyclosporins of primary-active ATP-dependent transporters in the hepatocyte canalicular membrane. FEBS Lett. 333:193–196 (1993).
M. Böhme, M. Müller, I. Leier, G. Jedlitschky, and D. Keppler. Cholestasis caused by inhibition of the adenosine triphosphate-dependent bile salt transport in rat liver. Gastroenterology 107:255–265 (1994).
R. Evers, M. Kool, L. van Deemter; H. Janssen, J. Calafat, L. C. J. M. Oomen, C. C. Paulusma, R. P. J. Oude Elferink, F. Baas, A. H. Schinkel, and P. Borst. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J. Clin. Invest. 101:1310–1319 (1998)
T. P. Schaub, J. Kartenbeck, J. König, O. Vogel, R. Witzgall, W. Kriz, and D. Keppler. Expression of the conjugate export pump encoded by the mrp2. J. Am. Soc. Nephrol. 8:1213–1221 (1997).
W. S. Pascoe, K. Inukai, Y. Oka, J. W. Slot, and D. E. James. Differential targeting of facilitative glucose transporters in polarized epithelial cells. Am. J. Physiol. 271:C547–554 (1996).
E. S. Quabius, H. Murer, and J. Biber. Expression of proximal tubular Na-Pi and Na-SO4 cotransporters in MDCK and LLCPK1 cells by transfection. Am. J. Physiol. 270:F220–228 (1996).
H. H. Gu, J. Ahn, M. J. Caplan, R. D. Blakely, A. I. Levey, and G. Rudnick. Cell-specific sorting of biogenic amine transporters expressed in epithelial cells. J. Biol. Chem. 271:18100–18106 (1996).
E. Camerer, S. Pringle, A. H. Skartlien, M. Wiiger, K. Prydz, A. B. Kolsto, and H. Prydz. Opposite sorting of tissue factor in human umbilical vein endothelial cells and Madin-Darby canine kidney epithelial cells. Blood 88:1339–1349 (1996).
C. Haass, E. H. Koo, D. B. Teplow, and D. J. Selkoe. Polarized secretion of beta-amyloid precursor protein and amyloid betapeptide in MDCK cells. Proc. Natl. Acad. Sci. USA 91:1564–1568 (1994).
P. Borst, M. Kool, and R. Evers. Do cMOAT (MRP2), other MRP homologues, and LRP play a role in MDR? Semin. Cancer Biol. 8:205–213 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kinoshita, S., Suzuki, H., Ito, K. et al. Transfected Rat cMOAT Is Functionally Expressed on the Apical Membrane in Madin-Darby Canine Kidney (MDCK) Cells. Pharm Res 15, 1851–1856 (1998). https://doi.org/10.1023/A:1011953906065
Issue Date:
DOI: https://doi.org/10.1023/A:1011953906065