Abstract
Purpose. The population PK/PD approach was prospectively used to determine the PK/PD of cisatracurium in various subgroups of healthy surgical patients.
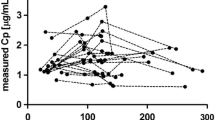
Methods. Plasma concentration (Cp) and neuromuscular block data from 241 patients in 8 prospectively-designed Phase I−III trials were pooled and analyzed using NONMEM. The analyses included limited Cp-time data randomly collected from 186 patients in efficacy/safety studies and full Cp-time data from 55 patients in pharmacokinetic studies. The effects of covariates on the PK/PD parameters of cisatracurium were evaluated. The time course of neuromuscular block was predicted for various patient subgroups.
Results. The population PK/PD model for cisatracurium revealed that anesthesia type, gender, age, creatinine clearance, and presence of obesity were associated with statistically significant (p < 0.01) effects on the PK/PD parameters of cisatracurium. These covariates were not associated with any clinically significant changes in the predicted recovery profile of cisatracurium. Slight differences in onset were predicted in patients with renal impairment and patients receiving inhalation anesthesia. Based on the validation procedure, the model appears to be accurate and precise.
Conclusions. The prospective incorporation of a population PK/PD strategy into the clinical development of cisatracurium generated information which influenced product labeling and reduced the number of studies needed during development.
Similar content being viewed by others
REFERENCES
C. Lee, B. K. Tran. In S. M. Rupp, R. R. Kirby, D. L. Brown (eds), Problems in Anesthesia Vol 3,No. 3, J. B. Lippincott Company, Maryland 1989, pp. 379–393.
S. Beal, L. B. Sheiner, eds. NONMEM user's guides 1992, NONMEM Project Group, University of California at San Francisco, San Francisco.
C. C. Peck, D. P. Conner, and M. G. Murphy. Bedside clinical pharmacokinetics. Applied Therapeutics, Inc. Revised edition, 1991:81.
D. Kisor, V. D. Schmith, W. A. Wargin, et al. Anesth Analg, 83:1065–71 (1996).
R. Welch, A. Brown, and R. Dahl. Clin Pharmacol Ther, 58:132–4 (1995).
L. B. Sheiner and S. L. Beal. J Pharmacokin Biopharm 9(4):503–11 (1981).
Currently approved labeling for Nimbex® Injection, DNA 20–551.
S. J. L. Riegelman and M. Rowland. J Pharm Sci 57(1):128–33 (1968).
E. I. Eger. The pharmacology of isoflurane. Br J Anesth 56:71S–99S (1984).
E. Ornstein, C. A. Lien, R. S. Matteo, et al. Anesthesiology 84:520–5 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmith, V.D., Fiedler-Kelly, J., Phillips, L. et al. Prospective Use of Population Pharmacokinetics/ Pharmacodynamics in the Development of Cisatracurium. Pharm Res 14, 91–97 (1997). https://doi.org/10.1023/A:1012015719694
Issue Date:
DOI: https://doi.org/10.1023/A:1012015719694