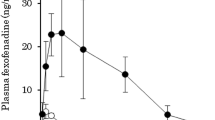
Abstract
Purpose. To investigate the relative contributions of the gut and liver to the first-pass loss of verapamil (VL) using anin vivo intestinal-vascular access port (IVAP) dog model.
Methods. Basic pharmacokinetics of VL were determined after intravenous (IV: 0.5 mg/kg), portal venous (PV: 2 mg/kg), and duodenal (ID: 2 mg/kg) administration in IVAP dogs. Serial blood samples were collected for 8 h after dosing, and plasma was analyzed for unchanged drug by a high-performance liquid chromatography-fluorescence method. Extraction ratios in the liver and intestinal tract were determined from the area under the concentration-time curves for ID, PV, and IV administration. The functional role of CYP450 or secretory transporters such as P-gp on the gut and liver first-pass loss of VL was further studied using ritonavir, a known substrate or inhibitor of these processes.
Results. The liver had a high intrinsic capacity for clearing VL because the absolute bioavailability (BA) of VL was 21.7% after PV administration. The BA of VL after ID administration was 23.5%; therefore, intestinal absorption was complete and intestinal extraction was negligible (ERGI ∼ 0). The BA of VL increased from 23.5% to 66.2% in the presence of ritonavir primarily due to a reduction in hepatic extraction.
Conclusions. Although the liver had a high intrinsic capacity for extracting VL, the contribution of gut to the first-pass loss of VL was negligible. Because of the additive effects of intestinal CYP3A-mediated metabolism and secretory transport, a significant gut first-pass effect was expected, but not observed in dogs. These studies demonstrate the utility of the in vivo IVAP dog model for evaluating the relative contribution of the gut and liver to the first-pass loss of drugs and for characterizing the functional role that CYP450 metabolism and/or secretory transporters play in drug-drug interactions and reduced oral bioavailability.
Similar content being viewed by others
REFERENCES
J. C. Kolars, W. M. Awni, R. M. Merion, and P. B. Watkins. First-pass metabolism of cyclosporine by the gut. Lancet 338:1488-1490 (1991).
K. E. Thummel, D.O'Shea, M. F. Paine, D. D. Shen, K. L. Kunze, J. D. Perkins, and G. R. Wilkinson. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin. Pharmacol. Ther. 59:491-502 (1996).
I. D. Waziers, P. H. Cugnenc, C. S. Yang, J.-P. Leroux, and P. H. Beaune. Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J. Pharmcol. Exp. Ther. 253:387-394 (1990).
C.-Y. Wu, L. Z. Benet, M. F. Hebert, S. K. Gupta, M. Rowland, D. Y. Gomez, and V. J. Wacher. Differentiation of absorption and first-pass gut and hepatic metabolism in humans: Studies with cyclosporine. Clin. Pharmacol. Ther. 58:492-497 (1995).
M. F. Paine, D. D. Shen, K. L. Kunze, J. D. Perkins, C. L. Marsh, J. P. McVicar, D. M. Barr, B. S. Gillies, and K. E. Thummel. First-pass metabolism of midazolam by the human intestine. Clin. Pharmacol. Ther. 60:14-24 (1996).
H. Saitoh and B. J. Aungst. Possible involvement of multiple P-glycoprotein-mediated efflux systems in the transport of verapamil and other organic cations across rat intestine. Pharm. Res. 12:1304-1310 (1995).
M. F. Fromm, D. Busse, H. K. Kroemer, and M. Eichelbaum. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology 24:796-801 (1996).
T. Terao, E. Hisanaga, Y. Sai, I. Tamai, and A. Tsuji. Active secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrier. J. Pharm. Pharmacol. 48:1083-1089 (1996).
Y. Zhang, X. Guo, E. T. Lin, and L. Z. Benet. Overlapping substrate specificities of cytochrome P450 3A and P-glycoprotein for a novel cysteine protease inhibitor. Drug Metab. Dispos. 26:360-366 (1998).
K. S. Lown, R. R. Mayo, A. B. Leichtman, H. L. Hsiao, D. K. Turgeon, P. Schmiedlin-Ren, M. B. Brown, W. Guo, S. J. Rossi, L. Z. Benet, and P. B. Watkins. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin. Pharmacol. Ther. 62:248-260 (1997).
P. J. Sinko, J. P. Sutyak, G. D. Leesman, P. Hu, V. Makhey, H. Yu, and C. L. Smith. Oral absorption of anti-AIDS nucleotide analogues: 3. Regional absorption and in vivo permeability of 2′,3′-dideoxyinosine in an intestinal-vascular access port (IVAP) dog model. Biopharm. Drug Dispos. 18:697-710 (1997).
P. J. Sinko, Y. H. Lee, V. Makhey, G. D. Leesman, J. P. Sutyak, H. Yu, B. Perry, C. L. Smith, P. Hu, E. J. Wagner, L. M. Falzone, L. T. McWhorter, J. P. Gilligan, and W. Stern. Biopharmaceutical approaches for developing and assessing oral peptide delivery strategies and systems: In vitro permeability and in vivo oral absorption of salmon calcitonin (sCT). Pharm. Res. 16:527-533 (1999).
H. Echizen and M. Eichelbaum. Clinical pharmacokinetics of verapamil, nifedipine and diltiazem. Clin. Pharmacokinet. 11:425-449 (1986).
V. D. Makhey, A. Guo, D. A. Norris, P. Hu, J. Yan, and P. J. Sinko. Characterization of the regional intestinal kinetics of drug efflux in rat and human intestine and in Caco-2 cells. Pharm. Res. 15:1160-1167 (1998).
J. P. Sutyak, Y. H. Lee, B. A. Perry, W. Stern, V. Makhey, and P. J. Sinko. Improved longevity and functionality of a canine model providing portal vein and multi-site intestinal access. Lab. Animal Sci. 50:68-75 (2000).
T. H. Arnold, R. L. Tackett, and J. J. Vallner. Pharmacodynamics of acute intranasal administration of verapamil: comparison with iv and oral administration. Biopharm. Drug Dispos. 6:447-454 (1985).
D. J. Kempf, K. C. Marsh, G. Kumar, A. D. Rodrigues, J. F. Denissen, E. McDonald, M. J. Kukulka, A. Hsu, G. R. Granneman, P. A. Baroldi, E. Sun, D. Pizzuti, J. J. Plattner, D. W. Norbeck, and J. M. Leonard. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob. Agents Chemother. 41:654-660 (1997).
G. N. Kumar, A. D. Rodrigues, A. M. Buko, and J. F. Denissen. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J. Pharmacol. Exp. Ther. 277:423-431 (1996).
J. Alsenz, H. Steffan, and R. Alex. Active apical secretary efflux of the HIV protease inhibitors saquinavir and ritonavir in Caco-2 monolayers. Pharm. Res. 15:423-428 (1998).
M. Gibaldi and D. Perrier. Pharmacokinetics, 2nd edn, Marcel Dekker, New York, 1982.
W. L. Chiou. Critical evaluation of potential error in pharmacokinetic studies using the linear trapezoidal rule method for the calculation of the area under the plasma level-time curve. J. Pharmacokin. Biopharm. 6:539-546 (1978).
G. M. Grass and P. J. Sinko. Effect of diverse datasets on the predictive capability of ADME models in drug discovery. Drug Discovery Today 6(HTS Suppl):158-165 (2001).
N. Zaman, S. Tawfik, D. Kwok, J. E. Axelson, H. Wiltshire, and Y. K. Tarn. Pharmacokinetics of saquinavir in the instrumented dogs. Pharm. Res. 1:S670 (1998).
S. R. Hamann, R. A. Blouin, and R. G. McAllister Jr. Clinical pharmacokinetics of verapamil. Clin. Pharmacokinet. 9:26-41 (1984).
M. F. Hebert. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv. Drug Deliv. Rev. 27:201-214 (1997).
N. Holtbecker, M. F. Fromm, H. K. Kroemer, E. F. Ohnhms, and H. Heidemann. The nifedipine-rifampin interaction: Evidence for induction of gut wall metabolism. Drug Metab. Dispos. 24:121-1123 (1996).
V. J. Wacher, J. A. Silverman, Y. Zhang, and L. Z. Benet. Role of P-glycoprotein and cytochrome P450 3A in limiting oral absorption of peptides and peptidomemetics. J. Pharm. Sci. 87:1322-1330 (1998).
S. D. Hall, K. E. Thummel, P. B. Watkins, K. S. Lown, L. Z. Benet, M. F. Paine, R. R. Mayo, D. K. Turgeon, D. G. Bailey, R. J. Fontana, and S. A. Wrighton. Molecular and physical mechanisms of first pass extraction. Drug Metab. Dispos. 27:161-166 (1999).
J. Hunter, B. H. Hirst, and N. L. Simmons. Drug absorption limited by P-glycoprotein-mediated secretary drug transport in human intestinal epithelial Caco-2 cell layers. Pharm. Res. 10:743-749 (1993).
J. Zacherl, G. Hamilton, T. Thalhammer, M. Riegler, E. P. Cosentini, A. Ellinger, G. Bischof, M. Schweitzer, B. Teleky, T. Koperna, and E. Wenzl. Inhibition of P-glycoprotein-mediated vinblastine transport across HCT-8 intestinal carcinoma monolayers by verapamil, cyclosporin A, and SDZ PSC833, in dependence on extracellular pH. Cancer Chemother. Pharmacol. 34:125-132 (1994).
M. F. Fromm, K. Dilger, D. Busse, H. K. Kroemer, M. Eichebaum, and U. Klotz. Gut wall metabolism of verapamil in older people: effects of rifampin-mediated enzyme induction. Br. J. Clin. Pharmacol. 45:247-255 (1998).
R. Sandstrom, A. Karlsson, L. Knutson, and H. Lennernas. Jejunal absorption and metabolism of R/S-verapamil in humans. Pharm. Res. 15:856-862 (1998).
W. L. Chiou, S. M. Chung, and T. C. Wu. Apparent lack of effect of P-glycoprotein on the gastrointestinal absorption of a substrate, tacrolimus, in normal mice. Pharm. Res. 17:205-208 (2000).
A. Sparreboom, J. van Asperen, U. Mayer, A. H. Schinkel, J. W. Smit, D. K.F. Meijer, P. Borst, W. J. Nooijen, J. H. Beijen, and O. van Tellingen. Limited oral bioavailability and active excretion of paclitaxel (Taxol) caused by P-glycoprotein in the intestine. Proc. Natl. Acad. Sci. USA 94:2031-2035 (1997).
U. Mayer, E. Wagenaar, J. H. Beijnen, J. W. Smit, D. K.W. Meijer, J. van Asperen, P. Borst, and A. H. Schinkel. Substantial excretion of digoxin via the intestinal mucosa and prevention of long-term digoxin accumulation in the brain by the mdr1a P-glycoprotein. Br. J. Pharmacol. 119:1038-1044 (1996).
M. T. Smith, M. J. Eadie, and T. O. Brophy. The pharmacokinetics of midazolam in man. Eur. J. Clin. Pharmacol. 19:271-278 (1981).
N. N. Vachharajani, W. C. Shyu, V. R. Shah, and R. H. Barbhaiya. Pharmacokinetic assessment of the sites of first-pass metabolism of BMS-181101, an antidepressant, in rats. J. Pharm. Pharmacol. 50:275-278 (1997).
S. Kumar, K. W. Riggs, and D. W. Rurak. Role of the liver and gut in systemic diphenhydramine clearance in adult nonpregnant sheep. Drug Metab. Dispos. 27:297-302 (1999).
C. H. Kleinbloesem, J. Van Hartan, J. P.H. Wilson, M. Danhof, P. Van Brummek, and D. D. Breimer. Nifedipine: Kinetics and hemodynamics effects in patients with liver cirrhosis after intravenous and oral administration. Clin. Pharmacol. Ther. 40:21-28 (1986).
M. Eichelbaum, M. Albrecht, G. Kliems, K. Schafer, and A. Somogyi. Influence of meso-caval shunt surgery on verapamil kinetics, bioavailability and response. Br. J. Clin. Pharmacol. 10:527-530 (1980).
J. H. Lin, M. Chiba, and T. Baillie. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol. Rev. 51:135-157 (1999).
W. D. Stern. Kinetics of the multidrug transporter (P-glycoprotein) and its reversal. Physiol. Rev. 77:545-590 (1997).
Y. H. Lee, M. H. Lee, and C. K. Shim. Decreased systemic clearance of diltiazem with increased hepatic metabolism in rats with uranyl nitrate-induced acute renal failure. Pharm. Res. 9:1599-1606 (1992).
H. K. Kroemer, J. C. Gautier, P. Beaune, C. Henderson, C. R. Wolf, and M. Eichelbaum. Identification of P450 enzymes involved in metabolism of verapamil in humans. Naunyn-Schmiedeberg's Arch. Pharmacol. 348:332-337 (1993).
M. T. Huisman, J. W. Smit, H. R. Wiltshire, R. M. Hoetelmans, J. H. Beijnen, and A. H. Achinkel. P-glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol. Pharmacol. 59:806-813 (2001).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, YH., Perry, B.A., Lee, HS. et al. Differentiation of Gut and Hepatic First-Pass Effect of Drugs: 1. Studies of Verapamil in Ported Dogs. Pharm Res 18, 1721–1728 (2001). https://doi.org/10.1023/A:1013374630274
Issue Date:
DOI: https://doi.org/10.1023/A:1013374630274