Abstract
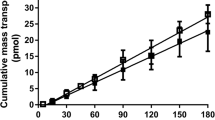
Caco-2 monolayers grown on Transwell polycarbonate membranes have been characterized as a valuable tool in drug transport studies. Despite the clear advantages of this system, the lack of stirring may create an unstirred water layer (UWL) whose resistance may limit the transcellular transport of lipophilic molecules. The objective of this study was to evaluate a novel diffusion cell where the transport buffer is mixed by gas lift and to determine the mixing flow rate needed to reduce the thickness (h) of the UWL adjacent to cell monolayers. The transport of the leakage marker, mannitol, remained at least 15-fold lower than the flux of testosterone, indicating that the stirring flow rates used did not affect the integrity of the monolayers. The permeability (P) of testosterone (log PC 3.13) across monolayers mounted on this diffusion cell was 4.07, 10.90, and 14.18 × 10−5 cm/sec at flow rates of 0, 15, and 40 ml/min, respectively, and the apparent UWLs were calculated to be 1966, 733, and 564µm. P and h in the stagnant Transwell were 3.08 × 10−5 cm/sec and 2597 µm, respectively. On the other hand, h was significantly smaller in the unstirred, cell-free membranes than in their cell-containing counterparts. P was correlated with lipophilicity and, in the case of the more lipophilic compounds, with the mixing flow rate.
Similar content being viewed by others
REFERENCES
K. L. Audus, R. L. Bartel, I. J. Hidalgo, and R. T. Borchardt. The use of cultured epithelial and endothelial cells for drug transport and metabolism studies. Pharm. Res. 7:435–451 (1990).
R. T. Borchardt, I. J. Hidalgo, K. M. Hillgren, and M. Hu. Pharmaceutical applications of cell culture. An overview. In G. Wilson, L. Illum, and S. S. Davies (eds.), Pharmaceutical Applications of Cell and Tissue Culture, Plenum, New York (in press).
M. Pinto, S. Robine-Leon, M.-D. Appay, M. Kedinger, N. Triadou, E. Dussaulx, B. Lacroix, P. Simon-Assmann, K. Haffen, J. Fogh, and A. Zweibaum. Enterocytic differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 47:323–330 (1983).
F. Raul, M. Kedinger, P. Simon, J. Grenier, and K. Haffen. Behaviour of isolated rat intestinal cells maintained in suspension or monolayer cultures. Biol. Cell 33:163–168 (1978).
A. Quaroni, J. Wands, R. L. Trelstad, and K. J. Isselbacher. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J. Cell Biol. 80:248–265 (1979).
M. Pinto, M.-D. Appay, P. Simon-Assman, G. Chevalier, N. Dracopoli, J. Fogh, and A. Zweibaum. Enterocytic differentiation of cultured human colon cancer cells by replacement of glucose by galactose in the medium. Biol. Cell 44:193–196 (1982).
I. J. Hidalgo, T. J. Raub, and R. T. Borchardt. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96:736–749 (1989).
P. Artursson. Epithelial transport of drugs in cell culture. I. A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79:476–482 (1990).
J. M. Dietschy and H. Westergaard. The effect of unstirred water layers on various transport processes in the intestine. In T. Z. Csaky (ed), Intestinal Absorption and Malabsorption, Raven, New York, 1975, pp. 197–206.
G. M. Grass and S. A. Sweetana. In vitro measurement of gastrointestinal tissue permeability using a new diffusion cell. Pharm. Res. 5:372–376 (1988).
I. Komiya, J. Y. Park, A. Kamani, N. F. Ho, and W. I. Higuchi. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int. J. Pharm. 4:249–262 (1980).
W. C. Schefler. Statistics for the Biological Sciences, Addison, Menlo-Park, 1979.
M. Heyman, R. Ducroc, J.-F. Desjeux, and J. L. Morgat. Horseradish peroxidase transport across adult rabbit jejunum in vitro. Am. J. Physiol. 242:G558–G564 (1982).
P. F. Curran and A. K. Solomon. Ion and water fluxes in the ileum of rats. J. Gen. Physiol. 4:143–168 (1957).
P. Artursson and C. Magnusson. Epithelial transport of drugs in cell culture. II. Effect of extracellular calcium (Ca2+) concentration on the paracellular transport of drugs of different lipophilicities across monolayers of intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 79:595–600 (1990).
I. J. Hidalgo, K. M. Hillgren, G. M. Grass, and R. T. Borchardt. A new side-by-side diffusion cell for studying transport across epithelial cell monolayers (submitted for publication).
F. A. Wilson and J. M. Dietschy. The intestinal unstirred layer: Its surface area and effect on active transport kinetics. Biochim. Biophys. Acta 363:112–126 (1974).
T. Levitt, T. Aufderheide, C. A. Fetzer, J. H. Bond, and D. G. Levitt. Use of carbon monoxide to measure luminal stirring in the rat gut. J. Clin. Invest. 74:2056–2064 (1984).
D. Winne. Unstirred layer, source of biased Michaelis constant in membrane transport. Biochim. Biophys. Acta 298:27–31 (1973).
A. B. R. Thomson. Limitations of the Eadie-Hofstee plot to estimate kinetic parameters of intestinal transport in the presence of an unstirred water layer. J. Membr. Biol. 47:39–57 (1979).
A. B. R. Thomson. Limitations of Michaelis-Menten kinetics in presence of intestinal unstirred layers. Am. J. Physiol. 236:E701–E709 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hidalgo, I.J., Hillgren, K.M., Grass, G.M. et al. Characterization of the Unstirred Water Layer in Caco-2 Cell Monolayers Using a Novel Diffusion Apparatus. Pharm Res 8, 222–227 (1991). https://doi.org/10.1023/A:1015848205447
Issue Date:
DOI: https://doi.org/10.1023/A:1015848205447