Abstract
Purpose. The purpose of this study was to investigate the pH-dependent passive and active transport of weakly basic drugs across the human intestinal epithelium.
Methods. The bidirectional pH-dependent transport of weak bases was studied in Caco-2 cell monolayers in the physiologic pH range of the gastrointestinal tract.
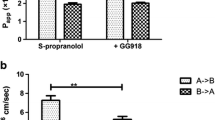
Results. A net secretion of atenolol and metoprolol was observed when a pH gradient was applied. However, the bidirectional transport of both compounds was equal in the nongradient system. Hence, at lower apical than basolateral pH a change in passive transport caused by an imbalance in the concentration of the uncharged drug species resulted in a “false” asymmetry (efflux ratio). Furthermore, a mixture of pH-dependent passive and active efflux was found for the P-glycoprotein (P-gp, MDR1, ABCB1) substrates, talinolol and quinidine, but not for the neutral drug, digoxin. However, the clinically important digoxin-quinidine interaction depended on the presence of a pH gradient. Hence, the degree of interaction depends on the amount of quinidine available at the binding site of the P-gp.
Conclusions. Active efflux of weak bases can only be accounted for when the fraction of unionized drug species is equal in all compartments because the transport is biased by a pH-dependent passive component. However, this component may take part in vivo and contribute to drug-drug interactions involving P-gp.
Similar content being viewed by others
REFERENCES
P. A. Shore, B. B. Brodie, and C. A. M. Hogben. The gastric secretion of drugs: A pH partition hypothesis. J. Pharmacol. Exp. Ther. 119:361-369 (1957).
J. Fallingborg, L. A. Christensen, M. Ingelman-Nielsen, B. A. Jacobsen, K. Abildgaard, and H. H. Rasmussen. pH-Profile and #x00AEional transit times of the normal gut measured by radiotelemetry device. Aliment. Pharmacol. Ther. 3:605-613 (1989).
G. T. McEwan and M. L. Lucas. The effect of E. coli STa enterotoxin on the absorption of weakly dissociable drugs from rat proximal jejunum in vivo. Br. J. Pharmacol. 101:937-943 (1990).
D. C. Taylor, R. Pownall, and W. Burke. The absorption of #x0392-adrenoceptor antagonists in rat in-situ small intestine; the effect of lipophilicity. J. Pharm. Pharmacol. 37:280-283 (1985).
A. Tsuji, E. Miyamoto, N. Hashimoto, and T. Yamana. GI absorption of Β-lactam antibiotics II: Deviation from pH-partition hypothesis in penicillin absorption through in situ and in vitro lipoidal barriers. J. Pharm. Sci. 67:1705-1711 (1978).
K. Palm, K. Luthman, J. Ros, J. Grasjo, and P. Artursson. Effect of molecular charge on intestinal epithelial drug transport: pH-dependent transport of cationic drugs. J. Pharmacol. Exp. Ther. 291:435-443 (1999).
J. C. Hardman, L. E. Limbird, and A. G. Gilman. Goodman Gilman's: The Pharmacological basis of Therapeutics, 10th Edition, McGraw-Hill, New York, 2001.
S. Yamashita, T. Furubayashi, M. Kataoka, T. Sakane, H. Sezaki, and H. Tokuda. Optimized conditions for prediction of intestinal drug permeability using Caco-2 cells. Eur. J. Pharm. Sci. 10:195-204 (2000).
M. Boisset, R. P. Botham, K. D. Haegele, B. Lenfant, and J. I. Pachot. Absorption of angiotensin II antagonists in Ussing chambers, Caco-2, perfused jejunum loop and in vivo: importance of drug ionisation in the in vitro prediction of in vivo absorption. Eur. J. Pharm. Sci. 10:215-224 (2000).
K. Palm, K. Luthman, A. L. Ungell, G. Strandlund, F. Beigi, P. Lundahl, and P. Artursson. Evaluation of dynamic polar molecular surface area as predictor of drug absorption: comparison with other computational and experimental predictors. J. Med. Chem. 41:5382-5392 (1998).
M. F. Fromm, R. B. Kim, C. M. Stein, G. R. Wilkinson, and D. M. Roden. Inhibition of P-glycoprotein-mediated drug transport: A unifying mechanism to explain the interaction between digoxin and quinidine. Circulation 99:552-557 (1999).
K. Westphal, A. Weinbrenner, T. Giessmann, M. Stuhr, G. Franke, M. Zschiesche, R. Oertel, B. Terhaag, H. K. Kroemer, and W. Siegmund. Oral bioavailability of digoxin is enhanced by talinolol: evidence for involvement of intestinal P-glycoprotein. Clin. Pharmacol. Ther. 68:6-12 (2000).
P. N. Craig. Drug compendium. In C. J. Drayton (Ed.), Cumulative Subject Index & Drug Compendium, Vol. 6, Pergamon Press, Oxford, 1990, pp. 237-991.
W. Kamm, J. Hauptmann, I. Behrens, J. StÜrzebecher, F. Dullweber, H. Gohlke, M. Stubbs, G. Klebe, and T. Kissel. Transport of Peptidomimetic Thrombin Inhibitors with a 3-Amino-Phenylalanine Structure: Permeability and Efflux Mechanism in Monolayers of a Human Intestinal Cell Line (Caco-2). Pharm. Res. 18:1110-1118 (2001).
R. A. Boyd, R. H. Stern, B. H. Stewart, X. Wu, E. L. Reyner, E. A. Zegarac, E. J. Randinitis, and L. Whitfield. Atorvastatin coadministration may increase digoxin concentrations by inhibition of intestinal P-glycoprotein-mediated secretion. J. Clin. Pharmacol. 40:91-98 (2000).
A. Rakhit, N. H. G. Holford, T. W. Guentert, K. Maloney, and S. Riegelman. Pharmacokinetics of quinidine and three of its metabolites in Man. J. Pharmacokinet. Biopharm. 12:1-21 (1984).
B. Terhaag, U. Palm, H. Sahre, K. Richter, and R. Oertel. Interaction of talinolol and sulfasalazine in the human gastrointestinal tract. Eur. J. Clin. Pharmacol. 42:461-462 (1992).
P. Artursson. Epithelial transport of drugs in cell culture. I: A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. J. Pharm. Sci. 79:476-482 (1990).
J. Hunter, B. H. Hirst, and N. L. Simmons. Epithelial secretion of vinblastine by human intestinal adenocarcinoma cell (HCT-8 and T84) layers expressing P-glycoprotein. Br. J. Cancer 64:437-444 (1991).
A. Adson, P. S. Burton, T. J. Raub, C. L. Barsuhn, K. L. Audus, and N. F. H. Ho. Passive diffusion of weak organic electrolytes across Caco-2 cell monolayers: Uncoupling the contributions of hydrodynamic, transcellular, and paracellular barriers. J. Pharm. Sci. 84:1197-1204 (1995).
A. Adson, T. J. Raub, P. S. Burton, C. L. Barsuhn, A. R. Hilgers, K. L. Audus, and N. F. H. Ho. Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers. J. Pharm. Sci. 83:1529-1536 (1994).
F. Wohnsland and B. Faller. High-throughput permeability pH profile and high-throughput alkane/water log P with artificial membranes. J. Med. Chem. 44:923-930 (2001).
D. Sun, H. Lennernas, L. S. Welage, J. L. Barnett, C. P. Landowski, D. Foster, D. Fleisher, K.-D. Lee, and G. L. Amidon. Comparison of human duodenum and Caco-2 gene expression profiles for 12,000 gene sequences tags and correlation with permeability of 26 drugs. Pharm. Res. 19:1400-1416 (2002).
T. GramattÉ, R. Oertel, B. Terhaag, and W. Kirch. Direct demonstration of small intestinal secretion and site-dependent absorption of the Β-blocker talinolol in humans. Clin. Pharmacol. Ther. 59:541-549 (1996).
T. W. Loo, M. C. Bartlett, and D. M. Clarke. The ‘LSGGQ’ Motif in Each Nucleotide-binding Domain of Human P-glycoprotein Is Adjacent to the Opposing Walker A Sequence. J. Biol. Chem. 277:41303-41306 (2002).
T. W. Loo and D. M. Clarke. Location of the rhodamine binding-site in the human multidrug resistance P-glycoprotein. J. Biol. Chem. 277:44332-44338 (2002).
R. Ohashi, I. Tamai, H. Yabuuchi, J. I. Nezu, A. Oku, Y. Sai, M. Shimane, and A. Tsuji. Na(+)-dependent carnitine transport by organic cation transporter (OCTN2): its pharmacological and toxicological relevance. J. Pharm. Exp. Ther. 291:778-784 (1999).
Y. Cui, J. Konig, and D. Keppler. Vectorial transport by double-transfected cells expressing the human uptake transporter SLC21A8 and the apical export pump ABCC2. Mol. Pharmacol. 60:934-943 (2001).
M. Sasaki, H. Suzuki, K. Ito, T. Abe, and Y. Sugiyama. Transcellular transport of organic anions across a double-transfected Madin-Darby canine kidney II cell monolayer expressing both human organic anion-transporting polypeptide (OATP2/SLC21A6) and Multidrug resistance-associated protein 2 (MRP2/ABCC2). J. Biol. Chem. 277:6497-6503 (2002).
I. A. M. de Lannoy and M. Silverman. The MDR1 gene product, P-Glycoprotein, mediates the transport of the cardiac Glycoside, Digoxin. Biochem. Biophys. Res. Commun. 189:551-557 (1992).
K. Goda, L. Balkay, T. Marian, L. Tron, A. Aszalos, and G. Szabo Jr. Intracellular pH does not affect drug extrusion by P-glycoprotein. J. Photochem. Photobiol. B 34:177-182 (1996).
G. A. Altenberg, G. Young, J. K. Horton, D. Glass, J. A. Belli, and L. Reuss. Changes in intra-or extracellular pH do not mediate P-glycoprotein-dependent multidrug resistance. Proc. Nati. Acad. Sci. USA 90:9735-9738 (1993).
E. Landwojtowicz, P. Nervi, and A. Seelig. Real-time monitoring of P-glycoprotein activation in living cells. Biochemistry 41:8050-8057 (2002).
T. Sakaeda, T. Nakamura, M. Horinouchi, M. Kakumoto, N. Ohmoto, T. Sakai, Y. Morita, T. Tamura, N. Aoyama, M. Hirai, M. Kasuga, and K. Okumura. MDR1 genotype-related pharmacokinetics of digoxin after single oral administration in healthy Japanese subjects. Pharm. Res. 18:1400-1404 (2001).
S. Hoffmeyer, O. Burk, O. von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Nati. Acad. Sci. USA 97:3473-3478 (2000).
M. Verschraagen, C. H. Koks, J. H. Schellens, and J. H. Beijnen. P-glycoprotein system as a determinant of drug interactions: the case of digoxin-verapamil. Pharmacol. Res. 40:301-306 (1999).
W. L. Chiou, C. Ma, S. M. Chung, and T. C. Wu. An alternative hypothesis to involvement of intestinal P-glycoprotein as the cause for digoxin oral bioavailability enhancement by talinolol. Clin. Pharmacol. Ther. 69:79-81 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neuhoff, S., Ungell, AL., Zamora, I. et al. pH-Dependent Bidirectional Transport of Weakly Basic Drugs Across Caco-2 Monolayers: Implications for Drug–Drug Interactions. Pharm Res 20, 1141–1148 (2003). https://doi.org/10.1023/A:1025032511040
Issue Date:
DOI: https://doi.org/10.1023/A:1025032511040