Abstract
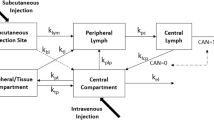
Purpose. The purpose of this work was to develop a pharmacokinetic model to describe the contribution of the lymphatics to the absorption and bioavailability of r-metHu-Leptin administered by subcutaneous (SC) injection to sheep.
Methods. r-metHu-Leptin was administered either by bolus intravenous injection (0.1 mg/kg) into the jugular vein or by SC injection (0.15 mg/kg) into the interdigital space of the hind leg. The SC groups included a non-cannulated control group and a lymph-cannulated group, in which peripheral lymph was continuously collected from a cannula in the efferent popliteal lymph duct. Serum and lymph concentrations were determined by enzyme-linked immunosorbent assay and profiles were modeled using compartmental pharmacokinetic methods. The fraction of the dose reaching the systemic circulation (F sys) and the proportions of the absorbed dose taken up via the blood (F blood) and lymph (F lymph) were determined.
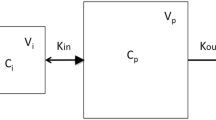
Results. Serum and lymph concentration vs. time profiles were well described by a two compartment model with parallel first order absorption into blood and lymph. F sys for the SC control group was 60.4 ± 8.4%. In the lymph-cannulated group, 21.7 ± 6.4% of the dose was recovered in serum and 34.4 ± 9.7% was recovered in peripheral lymph giving a total fraction absorbed (F abs) of 56.0 ± 10.3%. F sys for the SC control group was not significantly different to F abs in the lymph-cannulated group.
Conclusion. This study has shown that the lymph represents the predominant pathway for absorption of r-metHu-Leptin after SC administration.
Similar content being viewed by others
REFERENCES
M. A. Pelleymounter, M. J. Cullen, M. B. Baker, R. Hecht, D. Winters, T. Boone, and F. Collins. Effects of the obese gene product on body weight #x00AEulation in ob/ob mice. Science 269:540-543 (1995).
J. L. Halaas, K. S. Gajiwala, M. Maffei, S. L. Cohen, B. T. Chait, D. Rabinowitz, R. L. Lallone, S. K. Burley, and J. M. Friedman. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543-546 (1995).
A. Russell, A. Heatherington, M. McCamish, D. Lau, A. Watson, S. Mallard, S. Baughman, and S. Martin. Interspecies allometric predictions and pharmacokinetic comparisons of r-metHu-Leptin, a low molecular weight (16 kDa) protein hormone. In: Proceedings of the American Association of Pharmaceutical Scientists National Biotechnology Conference, San Diego, California, June 2002, pp. 103.
D. Lau, J. Lubina, R. M. Dixon, A. S. Greenberg, S. B. Heymsfield, K. Fujioka, R. Kushner, T. Hunt, J. Patane, and M. McCamish. Pharmacokinetics of recombinant methionyl human leptin (r-metHu-Leptin) and the effect of antibody formation in lean and obese subjects following subcutaneous (SC) dosing. In: Proceedings of the Eighth International Congress on Obesity, Paris, 1998, pp. HTP15.
F. Cumin, H. P. Baum, and N. Levens. Mechanism of leptin removal from the circulation by the kidney. J. Endocrinol. 155:577-585 (1997).
A. Supersaxo, W. R. Hein, and H. Steffen. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm. Res. 7:167-169 (1990).
S. A. Charman, A. M. S#x00C8, G. A. Edwards, and C. J. H. Porter. Systemic availability and lymphatic transport of human growth hormone administered by subcutaneous injection. J. Pharm. Sci. 89:168-177 (2000).
S. A. Charman, D. N. McLennan, G. A. Edwards, and C. J. H. Porter. Lymphatic absorption is a significant contributor to the subcutaneous bioavailability of insulin in a sheep model. Pharm. Res. 18:1620-1626 (2001).
A. Supersaxo, W. R. Hein, H. Gallati, and H. Steffen. Recombinant human interferon alpha-2a: delivery to lymphoid tissue by selected modes of application. Pharm. Res. 5:472-476 (1988).
C. J. H. Porter, G. A. Edwards, and S. A. Charman. Lymphatic transport of proteins after s.c injection: implications of animal model selection. Adv. Drug Deliv. Rev. 50:157-171 (2001).
L. V. Leak. Studies on the permeability of lymphatic capillaries. J. Cell Biol. 50:300-323 (1971).
E. Radwanski, A. Chakraborty, S. Van Wart, R. D. Huhn, D. L. Cutler, M. B. Affrime, and W. J. Jusko. Pharmacokinetics and leukocyte responses of recombinant human interleukin-10. Pharm. Res. 15:1895-1901 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McLennan, D.N., Porter, C.J.H., Edwards, G.A. et al. Pharmacokinetic Model to Describe the Lymphatic Absorption of r-metHu-Leptin After Subcutaneous Injection to Sheep. Pharm Res 20, 1156–1162 (2003). https://doi.org/10.1023/A:1025036611949
Issue Date:
DOI: https://doi.org/10.1023/A:1025036611949