Abstract
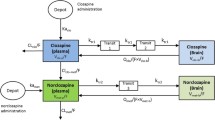
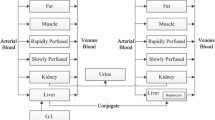
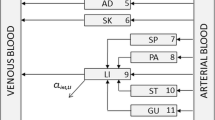
Three methods for estimation of the equilibrium tissue-to-plasma partition ratios (Kp values) in the presence of tissue concentration time data have been investigated. These are the area method, the open loop (tissue specific) method and the whole body model(closed loop) method, each with different model assumptions. Additionally, multiple imputations, a technique for dealing with deficiencies in data sets (i.e., missing tissues) is used. The estimated Kp values by the three methods have been compared and the limitations and advantages of each approach drawn. The area method, which is essentially model free, gives only a crude estimate of Kp without making any statement of its uncertainty; whereas both the open and closed loop methods provide an estimate of this. The closed loop method, where the most assumptions are made, is the approach that gives the best overall estimates of Kp, which was confirmed by comparing the predicted concentration–time profiles with experimental data. Although the estimates from the closed loop method, as well as the other two methods, are conditioned on the data, they are the most reliable for both propagating parameter variability and uncertainty through a whole body physiologically based model, as well as for extrapolation to human. A series of benzodiazepines, namely alprazolam, chlordiazepoxide, clobazam, diazepam, flunitrazepam, midazolam and triazolam in rat is used as a case study in the current investigation.
Similar content being viewed by others
REFERENCES
J. Mordenti. Man versus beast: Pharmacokinetic scaling in mammals. J. Pharm. Sci. 75: 1028–1040 (1986).
L. E. Gerlovski and R. K. Jain. Physiologically based pharmacokinetic modeling: Principles and applications. J. Pharm. Sci. 72: 1103–1129 (1983).
A. Bernareggi and M. Rowland. Physiological modeling of cyclosporine kinetics in rat and man. J. Pharmacokin. Biopharm. 19: 21–50 (1991).
S. Bjorkman, D. R. Stanski, H. Harashima, R. Dowrie, S. R. Harapat, D. R. Wada, and W. F. Ebling. Tissue distribution of fentanyl and alfentanil in the rat cannot be described by a blood flow limited model. J. Pharmacokin. Biopharm. 21: 255–279 (1993).
S. Bjorkman, D. R. Wada, D. R. Stanski and W. F. Ebling. Comparative pharmacoki-netics of fentanyl and alfentanil in rats and humans based on parametric single-tissue models. J. Pharmacokin. Biopharm. 22: 381–410 (1994).
R. Kawai, M. Lemaire, J.-L. Steimer, A. Bruelisauer, W. Niederberger, and M. Row-land. Physiologically based pharmacokinetic study on a cyclosporine derivative, SDZ IMM 125. J. Pharmacokin. Biopharm. 22: 327–365 (1994).
W. F. Ebling, D. R. Wada, and D. R. Stanski. From piecewise to full pharmacokinetic modeling: Application to thiopental disposition in the rat. J. Pharmacokin. Biopharm. 22: 259–292 (1994).
F. Marcucci, A. Guaitani, J. Kvetina, E. Mussini, and S. Garattini. Species difference in diazepam metabolism and anticonvulsant effect. Eur. J. Pharmacol. 4: 467–470 (1968).
F. Marcucci, R. Fanelli, E. Mussini, and S. Garattini. Further studies on species differ-ences in Diazepam metabolism. Eur. J. Pharmacol. 9: 253–256 (1970).
U. Klotz, K.-H. Antonin, and P. R. Bieck. Pharmacokinetics and plasma binding of diazepam in man, dog, rabbit, guinea pig and rat. J. Pharmacol. Exp. Ther. 199: 67–73 (1976).
S. Caccia, M. Carli, S. Garattini, E. Poggesi, R. Rech, and R. Samanin. Pharmacological activities of clobazam and diazepam in the rat: Relation to drug brain levels. Arch. Int. Pharmacodyn. 243: 275–283 (1980).
Y. Igari, Y. Sugiyama, Y. Sawada, T. Iga, and M. Nanano. Tissue distribution of 14C-diazepam and its metabolites in rats. Drug. Metab. Dispos. 10: 676–679 (1982).
Y. Igari, Y. Sugiyama, Y. Sawada, T. Iga, and M. Nanano. In vitro and in vivo assess-ment of hepatic and extrahepatic metabolism of diazepam in the rat. J. Pharm. Sci. 73: 826–828 (1984).
A. Becherucci, M. Palmi, and G. Segre. Pharmacokinetics of flunitrazepam in rats stud-ied by a radioreceptor assay. Pharmacol. Res. Commun. 17: 733–747 (1985).
L. Aarons, J. W. Mandema, and M. Danhof. A population analysis of the pharmacoki-netics and pharmacodynamics of midazolam in the rat. J. Pharmacokin. Biopharm. 19: 485–496 (1991).
J. W. Mandema, L. N. Sansom, M. Carmen Dios-Vieitez, M. Hollander-Jansen, and M. Danhof. Pharmacokinetic-Pharmacodynami modeling of the encephalographic effects of benzodiazepines. Correlation with receptor binding and anticonvulsant activity. J. Pharmacol. Exp. Ther. 257: 472–478 (1991).
J. W. Mandema, E. Tucker, and M. Danhof. Pharmacokinetic-pharmacodynamic model-ling of the EEG effects of midazolam in individual rats: influence of rate and route of administration. Br. J. Pharmacol. 102: 663–668 (1991).
X. Xie, S. H. Steiner, and M. H. Bickel. Kinetics of distribution and adipose tissue stor-age as a function of lipophilicity and chemical structure: II. Benzodiazepines. Drug. Metab. Dispos. 19: 15–19 (1991).
W. R. Banks, H. Yamakita, and G. A. Digenis. Metabolism and distribution of 1-[14C ] alprazolam in rats. J. Pharm. Sci. 81: 797–801 (1992).
J. M. Diaz-Garcia, J. Oliver-Botana, and D. Fos-Galve. Pharmacokinetics of diazepam in the rat: Influence of a carbon tetrachloride-induce hepatic injury. J. Pharm. Sci. 81: 768–772 (1992).
S. Hovinga, A. M. Stijnen, M. W. E. Langemeijer, J. W. Mandema, C. F. A. van Bezooijen, and M. Danhof. Pharmacokinetic-Eeg effect relationship of midazolam in aging BN/BiBij rats. Br. J. Pharmacol. 107: 171–177 (1992).
C. M. Hughes, J. G. Swanton, and P. S. Collier. The effect of three H2-receptor antago-nists on the disposition of midazolam in the rat in-situ perfused liver model. J. Pharm. Pharmacol. 46: 1029–1031 (1994).
K. Zomorodi, D. J. Carlile, and J. B. Houston. Kinetics of diazepam metabolism in rat hepatic microzomes and hepatocytes and their use in predicting in vivo hepatic clearance. Xenobiotica 9: 907–916 (1995).
S. Bjorkman, A. Fyge, and Z. Qi. Determination of the steady state tissue distribution of midazolam in the rat. J. Pharm. Sci. 85: 887–889 (1996).
J. Gaudreault, F. Varin, and G. M. Pollack. Anticonvulsant pharmacodynamics and dis-position of triazolam in rats. J. Pharm. Sci. 85: 999–1004 (1996).
A. Hoogerkamp, R. H. G. P. Arends, A. M. Bomers, J. W. Mandema, R. A. Voskuyl, and M. Danhof. Pharmacokinetic/pharmacodynamic relationship of benzodiazepines in the direct cortical simulation model of anticonvulsant effect. J. Phrmacol. Exp. Ther. 279: 803–812 (1996).
J. Schmider, D. J. Greenblatt, L. L. von Moltke, J. S. Harmatz, S. X. Duan, D. Karsov, and R. I. Shader. Characterization of six in vitro reactions mediated by human cyto-chrome P450: application to the testing of cytochrome P450-directed antibodies. Pharmacology 52: 125–134 (1996).
C. E. Lau and A. Heatherington. Pharmacokinetic-pharmacodynamic modeling of stim-ulatory and sedative effects of alprazolam: timing performance de cits. J. Pharmacol. Exp. Ther. 283: 1119–1129 (1997).
C. E. Lau, Y. Wang, and J. F. Falk. Differential reinforcement of low rate performance, pharmacokinetics and pharmacokinetic-pharmacodynamic modeling: independent inter-action of alprazolam and caffeine. J. Pharmacol. Exp. Ther. 281: 1013–1029 (1997).
A. Cleton, R. A. Voskuyl, and M. Danhof. Adaptive change in the pharmacodynamics of midazolam in different experimental modes of epilepsy: Kindling, cortical simulation and genetic absence epilepsy. Br. J. Pharmacol. 125: 615–620 (1998).
C. E. Lau, Y. Wang, and F. Ma. Pharmacokinetic-pharmacodynamic modeling of the coexistence of stimulatory and sedative components for midazolam. Eur. J. Pharmacol. 346: 131–144 (1998).
A. Cleton, D. Mazee, R. A. Voskuyl, and M. Danhof. Rate of change of blood concen-trations is a major determinant of the pharmacodynamics in rats. Br. J. Pharmacol. 127: 227–235 (1999).
F. Higashikawa, T. Murakami, T. Kaneda, and M. Takano. In vivo and in vitro meta-bolic clearance of midazolam, a cytochrome P450 3A substrate, by the liver under nor-mal and increased enzyme activity in rats. J. Pharm. Pharmacol. 51: 405–410 (1999).
Y. Wang, A. Roy, L. Sun, and C. E. Lau. A double-peak phenomenon in the pharmaco-kinetics of alprazolam after oral administration. Drug Metab Dispos. 27: 855–859 (1999).
L. Aarons, S. Toon, and M. Rowland. Validation of assay methodology used in pharma-cokinetic studies. J. Pharmacol. Meth. 17: 337–346 (1987).
F. D. Bondinot and W. J. Jusko. Fluid shifts and other factors affecting plasma protein binding of prednisolone by equilibrium dialysis. J. Pharm. Sci. 73 (6): 774–780 (1984).
S. L. Beal and L. Sheiner. The NONMEM system. Am. Stat. 34: 118–119 (1980).
Y. Igari, Y. Sugiyama, Y. Sawada, T. Iga, and M. Hanano. Prediction of diazepam dis-position in the rat and man by a physiologically based pharmacokinetic model. J. Phar-macokin. Biopharm. 11: 577–593 (1983).
I. Kuwahira, I. Gonzalez, N. Heisler, and J. Piper. Regional blood flows in conscious resting rats determined by microsphere distribution. J. Appl. Physiol. 74: 203–210 (1993).
D. M. Foster. Developing and testing integrated multicompartment models to describe a single-input multiple-output study using the SAAM II software system. Adv. Exp. Med. Biol. 445: 59–78 (1998).
ACSL Optimize User 's Guide. Version 1 for Windows. MGA Software. Concord MA, USA, 1996.
R. J. Carroll and D. Ruppert. Transformations and Weighting in Regression. Chapman and Hall, London, 1988.
MATLAB User 's guide version 6. 1. The MathWorks, UK.
J. L. Schafer. Analysis of incomplete multivariate data. Monographs on Statistics and Applied Probability, vol. 72, Chapman and Hall, London, 2000.
J. L. Shafer. Multiple imputation: a primer. Stat. Meth. Med. Res. 8: 3–15 (1999).
KOWWIN. Octanol-Water Partition Coefficient Program. User 's Guide, Syracuse Research Corporation, Merill Lane, Siracuse, NY, 1996.
M. D. Reed, C. M. Myers, and J. L. Blumer. Influence of midazolam on the protein binding of ketorolac. Curr. Therap. Res. Clin. Exp. 62: 558–565 (2001).
J. M. Gallo, F. C. Lam, and D. G. Perrier. Area method for the estimation of partition coefficients for physiological pharmacokinetic models. J. Pharmacokin. Biopharm. 15: 271–280 (1987)
H.-S. G. Chen and J. P. Gross. Estimation of tissue-to-plasma partition coefficients used in physiological pharmacokinetic models. J. Pharmacokin. Biopharm. 7: 117–125 (1979).
C. Tanaka, R. Kawai and M. Rowland. Physiologically based pharmacokinetics of cyclo-sporine A: Reevaluation of dose-nonlinear kinetics in rats. J. Pharmacokin. Biopharm. 27 (6): 597–623 (1999).
K. S. Pang and M. Rowland. Hepatic clearance of drugs. I. Theoretical considerations of a ''wellstirred ''model and a ''parallel tube ''model. Influence of hepatic blood flow plasma and blood cell binding and the hepatocellular enzymatic activity on hepatic drug clearance. J. Pharmacokin. Biopharm 5: 625–653 (1978).
M. Andersen, H. Clewell, M. Gargas, F. Smith, and R. Reitz. Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol. Appl. Pharmacol. 87: 187–205.
F. Bois, L. Zeise, and T. Tozer. Precision and sensitivity of pharmacokinetic models for cancer risk assessment: Tetrachlorethylene in mice, rats and humans. Toxicol. Appl. Pharmacol. 102: 300–315.
G. E. Blakey, I. A. Nestorov, P. A. Arundel, L. J. Aarons, and M. Rowland. Quantita-tive structure-pharmacokinetics relationships: I. Development of a whole-body physio-logically based model to characterize changes in pharmacokinetics across a homologous series of barbiturates in the rat. J. Pharmacokin. Biopharm. 25: 277–312 (1997).
Gueorguieva I. PhD thesis, University of Manchester, UK.
A. Dempster, N. Laird, and D. Rubin. Maximum likelihood estimation from incomplete data via the EM algorithm (with discussion). J. Roy. Stat. Soc. B 39: 1–38 (1977).
J. M. Scavone, H. Friedman, D. J. Greenblatt, and R. I. Shader. Effect of age, body composition, and lipid solubility on benzodiazepine tissue distribution in rats. Arzneim.-Forsch. Arzneim.-Forsch. (Drug Res. ) 37: 2–6 (1987).
P. Poulin and F.-P. Thiel. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J. Pharm. Sci. 91 (1): 129–156 (2002).
P. Ballard, D. E. Leahy, and M. Rowland. Prediction of in vivo tissue distribution from in vitro data 3. Correlation between in vitro and in vivo tissue distribution of a homolo-gous series of nine 5-n-alkyl-5-ethyl barbituric acid derivatives. Pharm. Res. 20: 864–872 (2003).
M. Easterling, M. Evans, and E. Kenyon. Comparative analysis of software for physio-logically based pharmacokinetic modelling: Simulation, optimisation and sensitivity anal-ysis. Toxicol. Meth. 10: 203–229 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gueorguieva, I., Nestorov, I.A., Murby, S. et al. Development of a Whole Body Physiologically Based Model to Characterise the Pharmacokinetics of Benzodiazepines. 1: Estimation of Rat Tissue-Plasma Partition Ratios. J Pharmacokinet Pharmacodyn 31, 269–298 (2004). https://doi.org/10.1023/B:JOPA.0000042737.14033.c6
Issue Date:
DOI: https://doi.org/10.1023/B:JOPA.0000042737.14033.c6