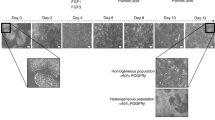
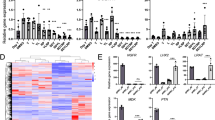
Abstract
Cell-based therapies hold the potential to alleviate the growing burden of liver diseases. Such therapies require human hepatocytes, which, within the stromal context of the liver, are capable of many rounds of replication. However, this ability is lost ex vivo, and human hepatocyte sourcing has limited many fields of research for decades. Here we developed a high-throughput screening platform for primary human hepatocytes to identify small molecules in two different classes that can be used to generate renewable sources of functional human hepatocytes. The first class induced functional proliferation of primary human hepatocytes in vitro. The second class enhanced hepatocyte functions and promoted the differentiation of induced pluripotent stem cell–derived hepatocytes toward a more mature phenotype than what was previously obtainable. The identification of these small molecules can help address a major challenge affecting many facets of liver research and may lead to the development of new therapeutics for liver diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shepard, C.W., Finelli, L. & Alter, M. Global epidemiology of hepatitis C virus infection. Lancet Infect. Dis. 5, 558–567 (2005).
Lin, H.M. et al. Center-specific graft and patient survival rates—1997 United Network for Organ Sharing (UNOS) Report. J. Am. Med. Assoc. 280, 1153–1160 (1998).
Allen, J.W., Hassanein, T. & Bhatia, S.N. Advances in bioartificial liver devices. Hepatology 34, 447–455 (2001).
Nelson, D.R. Cytochrome P450 and the individuality of species. Arch. Biochem. Biophys. 369, 1–10 (1999).
Gibbs, R.A. et al. Genome sequence of the Brown Norway rat yields insights into mammalian evolution. Nature 428, 493–521 (2004).
Mitaka, T. The current status of primary hepatocyte culture. Int. J. Exp. Pathol. 79, 393–409 (1998).
Runge, D., Michalopoulos, G.K., Strom, S.C. & Runge, D.M. Recent advances in human hepatocyte culture systems. Biochem. Biophys. Res. Commun. 274, 1–3 (2000).
Richman, R.A., Claus, T.H., Pilkis, S.J. & Friedman, D.L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc. Natl. Acad. Sci. USA 73, 3589–3593 (1976).
Shimaoka, S., Nakamura, T. & Ichihara, A. Stimulation of growth of primary cultured adult rat hepatocytes without growth factors by coculture with nonparenchymal liver cells. Exp. Cell Res. 172, 228–242 (1987).
Mitaka, T., Sattler, C.A., Sattler, G.L., Sargent, L.M. & Pitot, H.C. Multiple cell cycles occur in rat hepatocytes cultured in the presence of nicotinamide and epidermal growth factor. Hepatology 13, 21–30 (1991).
Block, G.D. et al. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGFα in a chemically defined (HGM) medium. J. Cell. Biol. 132, 1133–1149 (1996).
Mizuguchi, T. et al. Enhanced proliferation and differentiation of rat hepatocytes cultured with bone marrow stromal cells. J. Cell. Physiol. 189, 106–119 (2001).
Uyama, N. et al. Regulation of cultured rat hepatocyte proliferation by stellate cells. J. Hepatol. 36, 590–599 (2002).
Kobayashi, N. et al. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science 287, 1258–1262 (2000).
Castell, J.V., Jover, R., Martinez-Jimenez, C.P. & Gomez-Lechon, M.J. Hepatocyte cell lines: their use, scope and limitations in drug metabolism studies. Expert Opin. Drug Metab. Toxicol. 2, 183–212 (2006).
Nyberg, S.L. et al. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann. Surg. 220, 59–67 (1994).
Song, Z. et al. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 19, 1233–1242 (2009).
Si-Tayeb, K. et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305 (2010).
Sullivan, G.J. et al. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology 51, 329–335 (2010).
Ploss, A. et al. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc. Natl. Acad. Sci. USA 107, 3141–3145 (2010).
Khetani, S.R. & Bhatia, S.N. Microscale culture of human liver cells for drug development. Nat. Biotechnol. 26, 120–126 (2008).
Chen, A.A. et al. Humanized mice with ectopic artificial liver tissues. Proc. Natl. Acad. Sci. USA 108, 11842–11847 (2011).
Bhatia, S.N., Balis, U.J., Yarmush, M.L. & Toner, M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 13, 1883–1900 (1999).
Khetani, S. & Bhatia, S.N. Development and characterization of microscale models of rat and human livers. Hepatology 46, 773A (2007).
Carpenter, A.E. et al. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 7, R100 (2006).
Jones, T.R. et al. CellProfiler Analyst: data exploration and analysis software for complex image-based screens. BMC Bioinformatics 9, 482 (2008).
Jones, T.R. et al. Scoring diverse cellular morphologies in image-based screens with iterative feedback and machine learning. Proc. Natl. Acad. Sci. USA 106, 1826–1831 (2009).
Goessling, W. et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 136, 1136–1147 (2009).
North, T.E. et al. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc. Natl. Acad. Sci. USA 107, 17315–17320 (2010).
Michalopoulos, G.K. & DeFrances, M.C. Liver regeneration. Science 276, 60–66 (1997).
Michalopoulos, G.K. Liver regeneration. J. Cell. Physiol. 213, 286–300 (2007).
Higgins, G.M. & Anderson, R.M. Experimental pathology of liver: restoration of liver in white rat following partial surgical removal. Arch. Pathol. (Chic) 12, 186–202 (1931).
Overturf, K. et al. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat. Genet. 12, 266–273 (1996).
Rhim, J.A., Sandgren, E.P., Degen, J.L., Palmiter, R.D. & Brinster, R.L. Replacement of diseased mouse liver by hepatic cell transplantation. Science 263, 1149–1152 (1994).
Stöcker, E., Wullstein, H.K. & Bräu, G. Capacity of regeneration in liver epithelia of juvenile, repeated partially hepatectomized rats. Autoradiographic studies after continuous infusion of 3H-thymidine. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 14, 93–103 (1973).
Chen, S.B., Hilcove, S. & Ding, S. Exploring stem cell biology with small molecules. Mol. Biosyst. 2, 18–24 (2006).
North, T.E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007).
Wang, W. et al. Identification of small-molecule inducers of pancreatic β-cell expansion. Proc. Natl. Acad. Sci. USA 106, 1427–1432 (2009).
Michalopoulos, G. et al. Liver regeneration studies with rat hepatocytes in primary culture. Cancer Res. 42, 4673–4682 (1982).
Touboul, T., Vallier, L. & Weber, A. Robust differentiation of fetal hepatocytes from human embryonic stem cells and iPS. Med. Sci. (Paris) 26, 1061–1066 (2010).
Kang, L., Wang, J., Zhang, Y., Kou, Z. & Gao, S. iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 5, 135–138 (2009).
Zhao, X.Y. et al. iPS cells produce viable mice through tetraploid complementation. Nature 461, 86–90 (2009).
Azuma, H. et al. Robust expansion of human hepatocytes in Fah−/−/Rag2−/−/Il2rg−/− mice. Nat. Biotechnol. 25, 903–910 (2007).
Payne, C.M. et al. Persistence of functional hepatocyte-like cells in immune-compromised mice. Liver Int. 31, 254–262 (2011).
Liu, H., Kim, Y., Sharkis, S., Marchionni, L. & Jang, Y.-Y. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci. Transl. Med. 3, 82ra39 (2011).
Jozefczuk, J., Prigione, A., Chavez, L. & Adjaye, J. Comparative analysis of human embryonic stem cell and induced pluripotent stem cell–derived hepatocyte-like cells reveals current drawbacks and possible strategies for improved differentiation. Stem Cells Dev. 20, 1259–1275 (2011).
Behbahan, I.S. et al. New approaches in the differentiation of human embryonic stem cells and induced pluripotent stem cells toward hepatocytes. Stem Cell Rev. 7, 748–759 (2011).
Kroon, E. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 26, 443–452 (2008).
Acknowledgements
This research was funded by a Broad Institute Scientific Planning and Allocation of Resources Committee grant (S.N.B.), US National Institutes of Health (NIH) grants NIH R01-DK065152 (S.N.B.), NIH R01-DK56966 (S.N.B.) and NIH R01 GM089652 (A.E.C.), National Science Foundation CAREER award DBI 1148823 (A.E.C.) and National Institute of Diabetes and Digestive and Kidney Diseases grants DK085445 (W.G.), DK55743 (S.A.D.), DK087377 (S.A.D.), HL094857 (S.A.D.) and HG006398 (S.A.D.). S.N.B. is a Howard Hughes Medical Institute investigator. We thank H. Fleming for help with manuscript preparation, T. Golub and R. Gould for discussions and H. Green (Harvard University) for providing J2-3T3 fibroblasts. The authors dedicate this paper to the memory of Officer Sean Collier, for his caring service to the MIT community and his sacrifice.
Author information
Authors and Affiliations
Contributions
J.S. and S.N.B. designed all experiments. J.S. performed and analyzed all experiments. D.J.L. helped analyze image-based experiments. N.T.R. helped perform and analyze screening experiments and analyzed chemical characterization data. R.E.S. helped design, perform and analyze iPS experiments. D.T. developed and implemented the Luminex system. T.E.N., S.A.D., W.G. and A.E.C. contributed reagents and expertise. J.S., S.N.B. and N.T.R. wrote the manuscript. S.N.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results (PDF 4790 kb)
Rights and permissions
About this article
Cite this article
Shan, J., Schwartz, R., Ross, N. et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol 9, 514–520 (2013). https://doi.org/10.1038/nchembio.1270
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1270
This article is cited by
-
Peptide-based scaffolds for the culture and maintenance of primary human hepatocytes
Scientific Reports (2021)
-
HBV-related hepatocarcinogenesis: the role of signalling pathways and innovative ex vivo research models
BMC Cancer (2019)
-
Highly efficient and expedited hepatic differentiation from human pluripotent stem cells by pure small-molecule cocktails
Stem Cell Research & Therapy (2018)