Abstract
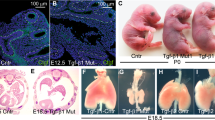
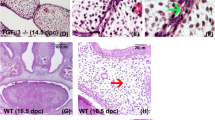
A broad spectrum of biological activities has been proposed for transforming growth factor–β3(TGF–β 3). To study TGF–β3function in development, TGF–β3 null mutant mice were generated by gene–targeting. Within 20 hours of birth, homozygous TGF–β3−/− mice die with unique and consistent phenotypic features including delayed pulmonary development and defective palatogenesis. Unlike other null mutants with cleft palate, TGF–β3−/− mice lack other concomitant craniofacial abnormalities. This study demonstrates an essential function for TGF–β3 in the normal morphogenesis of palate and lung, and directly implicates this cytokine in mechanisms of epithelial–mesenchymal interaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ten Dijke, P., Hansen, P., Iwata, K.K., Pieler, C. & Foulkes, J.G. Identification of another member of the transforming growth factor type β gene family. Proc. natn. Acad. Sci. U.S.A 85, 4715–4719 (1988).
Derynck, R. et al A new type of transforming growth factor-b, TGF-β3 EMBO J. 7, 3737–3743 (1988).
Massagué, J. The transforming growth factor-β family. Ann. Rev. Cell Biol. 6, 597–641 (1990).
Roberts, A.B. & Sporn, M.B. The transforming growth factor βs, In Peptide growth factors and their receptor: Handbook of Experimental Pharmacology (eds. Sporn, M.B. & Roberts, A.B.) 412–472 (Springer-Verlag, Heidelberg, 1990).
Graycar, J.L. et al. Human transforming growth factor (33: Recombinant expression, purification and biological activities in comparison with transforming growth factors-β1 and -β2. Molec. Endocrinol. 3, 1977–1986 (1989).
Miller, D.A., Pelton, R.W., Derynck, R. & Moses, H.L. Transforming growth factor-β. A family of growth regulatory peptide. Ann. N. Y. Acad. Sci. 593, 208–217 (1990).
Roberts, A.B. & Sporn, M.B. Differential expression of the TGF-β isoforms in embryogenesis suggests specific roles in developing and adult tissues. Molec. Reprod. Dev. 32, 91–98 (1992).
Schmid, P., Cox, D., Bilbe, G., Maier, R. & McMaster, G.K. Differential expression of TGF-β1, β2 and β3 genes during mouse embryogenesis. Development 111, 117–130 (1991).
Sharpe, P.M. & Ferguson, M.W.J. Mesenchymal influences on epithelial differentiation in developing systems. J. Cell Sci. Suppl. 10, 195–230 (1980).
Millan, F.A., Denhez, F., Kondaiah, P. & Akhurst, R.J. Embryonic gene expression patterns of TGF β1, β2 and β3 suggest different developmental functions in vivo. Development 111, 131–144 (1991).
Vorbroker, O.K., Profitt, S.A., Nogee, L.M., Whitsett, J.A. Aberrant processing of surfactant protein C in hereditary SP-B deficiency. Am. J. Physiol. 268, L647–656 (1995).
Pourtois, M. Onset of the acquired potentiality for fusion in the palatal shelves of rats. J. Embryol. exp. Morph. 16, 171–182 (1966).
Ferguson, M.W.J. Palate development. Development 103 Suppl., 41–60 (1988).
Greene, R.M. Signal transduction during craniofacial development. Grit. Rev. Toxicol. 20, 137–153 (1989).
Fitchett, J.E. & Hay, E.D. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev. Biol. 131, 455–474 (1989).
Shuler, C.F., Guo, Y., Majumder, A. & Luo, R. Molecular and morphologic changes during the epithelial-mesenchymal transformation of palatal shelf medial edge epithelium in vitro. Int. J. dev. Biol. 35, 463–472 (1991).
Shuler, C.F., Halpern, D.E., Quo, Y. & Sank, A.C. Medial edge epithelium fate traced by cell lineage analysis during epithelial-mesenchymal transformation in vivo. Dev. Biol. 154, 318–330 (1992).
Carette, M.J.M. & Ferguson, M.W.L. The fate of medial edge epithelial cells during palatal fusion in vitro: An analysis by Dil labeling and confocal microscopy. Development 114, 379–388 (1992).
Fitzpatrick, D.R., Denhez, F., kondaiah, P. & Akhurst, R. Differential expression of TGF-beta isoforms in murine palatogenesis. Development 109, 585–595 (1990).
Pelton, R.W., Hogan, B.L.M., Miller, D.A. & Moses, H.L. Differential expression of genes encoding TGFs β1, β2, and β3 during murine palate formation. Dev. Biol. 141, 456–460 (1990).
Brunet, C.L., Sharpe, P.M. & Ferguson, M.W.J. Inhibition of TGF-β3 (but not TGF-β1 or TGF-β2) activity prevents normal mouse embryonic palate fusion. Int. J. dev. Biol. 39, 345–355 (1995).
Shapiro, B.L. & Sweney, L.R. Electron microscopic and histochemical examination of oral epithelial-mesenchymal interaction (programmed cell death). J. Dent. Res. 48, 652–660 (1969).
Hudson, C.D. & Shapiro, B.L. A autoradiographic study of DMA synthesis in embryonic palatal shelf epithelium with reference to the concept of programmed cell death. Arch. Oral. Biol. 18, 77–84 (1973).
Pratt, R.M. & Martin, G.R. Epithelial cell death and cAMP increase during palatal development. Proc. natn. Acad. Sci. U.S.A. 72, 874–877 (1975).
Pratt, R.M. & Greene, R.M. Inhibition of palatal epithelial cell death by altered protein synthesis. Dev. Biol. 54, 135–145 (1976).
Potts, J.D., Dagle, J.M., Walder, J.A., Weeks, D.L. & Runyan, R.B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factors β3. Proc. natn. Acad. Sci. U.S.A. 88, 1516–1520 (1991).
Letterio, J.J. et al. Maternal rescue of transforming growth factor-β1 null mice. Science 264, 1936–1938 (1994).
Shannon, J.M. Induction of alveolar type II cell differentiation in foetal tracheal epithelium by grafted distal lung mesenchyme. Dev. Biol. 166, 600–614 (1994).
Minoo, P. & King, R.J. Epithelial-mesenchymal interactions in lung development. A Rev. Physiol. 56, 13–45 (1994).
Jetten, A.M., Volberg, T.M., Nervi, C. & George, M.D. Positive and negative regulation of proliferation and differentiation in tracheobronchial epithelial cells. An. Rev. resp. Dis. 142, S36–39 (1990).
Warburton, D. et al. Epigenetic role of epidermal growth factor expression and signalling in embryonic mouse lung morphogenesis. Dev. Biol. 149, 123–133 (1992).
Serra, R., Pelton, R.W. & Moses, H.L. TGF-β1 inhibits branching morphogenesis and N-myc expression in lung bud organ cultures. Development 120, 2153–2161 (1994).
Khalil, N. et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am. J. resp. Cell molec. Biol. 5, 155–162 (1991).
Denholm, E.M. & Rollins, S.M. Expression and secretion of transforming growth factor-beta by bleomycin-stimulated rat alveolar macrophages. Am. J. Physiol. 264, L36–42 (1993).
Rijli, F.M. et al. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75, 1333–1349 (1993).
Gendron-Maquire, M., Mallo, M., Zhang, M. & Gridley, T. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell 75, 1317–1331 (1993).
Satokata, I. & Maas, R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nature Genet. 6, 348–356 (1995).
Matzuk, M.M. et al. Functional analysis of activins during mammalian development. Nature 374, 354–356 (1994).
Matzuk, M.M. et al. Multiple defects and perinatal death in mice deficient in follistatin. Nature 374, 360–363 (1995).
Couture, L.A., Abbott, B.D. & Bimbaum, L.S. A critical review of the developmental toxicity and teratogenicity of 2,3,7,8-tetrachlorodi-benzo-p-dioxin: Recent advances towards understanding the mechanism. Teratology 42, 619–627 (1990).
Pratt, R.M., Dencker, L. & Diewert, V.M. 2,3,7,8 tetrachlorodibenzo-p-dioxin induced cleft palate in the mouse: evidence for alterations in palatal shelf fusion. Teratog. Carcinog. Mutagen. 4, 427–436 (1984).
Abbott, P.B., Perdew, G.H. & Birnbaum, L.S. Ah receptor in embryonic mouse palate and effects of TCDD on receptor expression. Tox. appl. Pharma. 126, 16–25 (1994).
Muglia, L., Jacobson, L., Dikkies, P. & Majzoub, R.A. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature 373, 427–432 (1995).
Wang, J. et al. Cloning and expression of glucocorticoid-induced genes in fetal rat lung fibroblasts. J. biol. Chem. 270, 2722–2728 (1995).
Smith, B.T. & Post, M. Tissue interactions in The Lung vol. 1 (eds. Crystal, R.G. & West, J.B.) Vol 1, 671–676 (Raven Press, New York, 1991).
Collabrative Group of Antenatal Steroid Therapy. Effects of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am. J. Obstet. Gynecol. 141, 276–287 (1981).
Pelton, R.W., Saxena, B., Jones, M., Moses, H.L. & Gold, L.I. Immunohistochemical localization of TGF-β1, TGF-β2 and TGF-β3 in mouse embryo: expression patterns suggest multiple roles during embryonic development. J. Cell Biol. 115, 1091–1105 (1991).
Miller, D.A. et al. Complementary DMA cloning of the murine transforming growth factor β3 (TGFβ3) precursor and the comparative expression of TGFβ3 and TGFβ1 messenger RNA in murine embryos and adult tissues. Molec. Endocrinol. 3, 1926–1934 (1989).
McLeod, J.M. Differential staining of cartilage and bone in whole mouse fetuses by Alcian blue and Alizarin red S. Teratology 22, 299–301 (1980).
Nagy, A., Rossant, J., Nagy, R., Abramow-Neweriy, W. & Roder, J.C. Derivatization of completely cell-culture-derived mice from early-passage embryonic stem cells. Proc. natn. Acad. Sci. U.S.A. 90, 8424–8428 (1993).
Wurst, W & Joyner, A Production of targeted embryonic stem cell clones in Gene Targeting: A Practical Approach (ed. Joyner, A.L.) 31–62 (Oxford Univ. Press, U.K., 1993).
Voncken, J.W et al. Increased neutrophil respiratory burst in BCR null mutants. Cell 80, 719–728 (1995).
Papaioannou, V.P & Johnson, R. Production of chimeras and genetically defined offspring from targeted ES cells In Gene Targeting: A Practical Approach. ed. Joyner, A.L.) 107–146 (Oxford Univ. Press, U.K., 1993).
Alonso, S, Minty, A, Bouriet, Y. & Buckingham, M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J. molec. Evol. 23, 11–22 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaartinen, V., Voncken, J., Shuler, C. et al. Abnormal lung development and cleft palate in mice lacking TGF–β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet 11, 415–421 (1995). https://doi.org/10.1038/ng1295-415
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ng1295-415
This article is cited by
-
The roles and regulatory mechanisms of TGF-β and BMP signaling in bone and cartilage development, homeostasis and disease
Cell Research (2024)
-
The Multifaceted Effects of Osteocytic TGFβ Signaling on the Skeletal and Extraskeletal Functions of Bone
Current Osteoporosis Reports (2023)
-
Specificity of TGF-β1 signal designated by LRRC33 and integrin αVβ8
Nature Communications (2022)
-
Targeting TGFβ signal transduction for cancer therapy
Signal Transduction and Targeted Therapy (2021)
-
FACEts of mechanical regulation in the morphogenesis of craniofacial structures
International Journal of Oral Science (2021)