Abstract
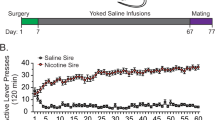
We delineated a heritable phenotype resulting from the self-administration of cocaine in rats. We observed delayed acquisition and reduced maintenance of cocaine self-administration in male, but not female, offspring of sires that self-administered cocaine. Brain-derived neurotrophic factor (Bdnf) mRNA and BDNF protein were increased in the medial prefrontal cortex (mPFC), and there was an increased association of acetylated histone H3 with Bdnf promoters in only the male offspring of cocaine-experienced sires. Administration of a BDNF receptor antagonist (the TrkB receptor antagonist ANA-12) reversed the diminished cocaine self-administration in male cocaine-sired rats. In addition, the association of acetylated histone H3 with Bdnf promoters was increased in the sperm of sires that self-administered cocaine. Collectively, these findings indicate that voluntary paternal ingestion of cocaine results in epigenetic reprogramming of the germline, having profound effects on mPFC gene expression and resistance to cocaine reinforcement in male offspring.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Anway, M.D., Cupp, A.S., Uzumcu, M. & Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005).
Dunn, G.A. & Bale, T.L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150, 4999–5009 (2009).
Champagne, F.A. Epigenetic influence of social experiences across the lifespan. Dev. Psychobiol. 52, 299–311 (2010).
Carone, B.R. et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096 (2010).
Ng, S.F. et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467, 963–966 (2010).
Morgan, C.P. & Bale, T.L. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. 31, 11748–11755 (2011).
Kaati, G., Bygren, L.O. & Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur. J. Hum. Genet. 10, 682–688 (2002).
Pembrey, M.E. et al. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14, 159–166 (2006).
Byrnes, E.M. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl.) 182, 537–544 (2005).
Byrnes, J.J., Babb, J.A., Scanlan, V.F. & Byrnes, E.M. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav. Brain Res. 218, 200–205 (2011).
Novikova, S.I. et al. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS One 3, e1919 (2008).
He, F., Lidow, I.A. & Lidow, M.S. Consequences of paternal cocaine exposure in mice. Neurotoxicol. Teratol. 28, 198–209 (2006).
Abel, E.L., Moore, C., Waselewsky, D., Zajac, C. & Russell, L.D. Effects of cocaine hydrochloride on reproductive function and sexual behavior of male rats and on the behavior of their offspring. J. Androl. 10, 17–27 (1989).
Burley, N. The differential-allocation hypothesis: an experimental test. Am. Nat. 132, 611–628 (1988).
Sheldon, T.A. & Smith, P.C. Equity in the allocation of health care resources. Health Econ. 9, 571–574 (2000).
Drickamer, L.C., Gowaty, P.A. & Holmes, C.M. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim. Behav. 59, 371–378 (2000).
Gowaty, P.A. et al. The hypothesis of reproductive compensation and its assumptions about mate preferences and offspring viability. Proc. Natl. Acad. Sci. USA 104, 15023–15027 (2007).
Alter, M.D. et al. Paternal transmission of complex phenotypes in inbred mice. Biol. Psychiatry 66, 1061–1066 (2009).
Martini, M. & Valverde, O. A single episode of maternal deprivation impairs the motivation for cocaine in adolescent mice. Psychopharmacology (Berl.) 219, 149–158 (2012).
Sadri-Vakili, G. et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J. Neurosci. 30, 11735–11744 (2010).
Berglind, W.J. et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur. J. Neurosci. 26, 757–766 (2007).
Pierce, R.C. & Bari, A.A. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev. Neurosci. 12, 95–110 (2001).
Cazorla, M. et al. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J. Clin. Invest. 121, 1846–1857 (2011).
Sakuma, Y. Gonadal steroid action and brain sex differentiation in the rat. J. Neuroendocrinol. 21, 410–414 (2009).
Koob, G.F. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 1314, 3–14 (2010).
McElligott, Z.A. & Winder, D.G. Modulation of glutamatergic synaptic transmission in the bed nucleus of the stria terminalis. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1329–1335 (2009).
Chung, W.C., Swaab, D.F. & De Vries, G.J. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J. Neurobiol. 43, 234–243 (2000).
Becker, J.B. & Hu, M. Sex differences in drug abuse. Front. Neuroendocrinol. 29, 36–47 (2008).
Anker, J.J. & Carroll, M.E. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr. Top. Behav. Neurosci. 8, 73–96 (2011).
Quinones-Jenab, V. & Jenab, S. Progesterone attenuates cocaine-induced responses. Horm. Behav. 58, 22–32 (2010).
Kendler, K.S., Jacobson, K.C., Prescott, C.A. & Neale, M.C. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am. J. Psychiatry 160, 687–695 (2003).
Tsuang, M.T. et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities. Arch. Gen. Psychiatry 55, 967–972 (1998).
Merikangas, K.R. et al. Familial transmission of substance use disorders. Arch. Gen. Psychiatry 55, 973–979 (1998).
Goldman, D., Oroszi, G. & Ducci, F. The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 6, 521–532 (2005).
Graham, D.L. et al. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci. 10, 1029–1037 (2007).
Whitfield, T.W. Jr., Shi, X., Sun, W.L. & McGinty, J.F. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J. Neurosci. 31, 834–842 (2011).
Maze, I. & Nestler, E.J. The epigenetic landscape of addiction. Ann. NY Acad. Sci. 1216, 99–113 (2011).
Misra, A.L., Giri, V.V., Patel, M.N., Alluri, V.R. & Mule, S.J. Disposition and metabolism of [3H] cocaine in acutely and chronically treated monkeys. Drug Alcohol Depend. 2, 261–272 (1977).
Li, H., George, V.K., Crossland, W.J., Anderson, G.F. & Dhabuwala, C.B. Characterization of cocaine binding sites in the rat testes. J. Urol. 158, 962–965 (1997).
Guerrero-Bosagna, C., Settles, M., Lucker, B. & Skinner, M.K. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE 5, e13100 (2010).
Steger, K., Cavalcanti, M.C. & Schuppe, H.C. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int. J. Androl. 34, 513–527 (2011).
Anway, M.D., Cupp, A.S., Uzumcu, M. & Skinner, M.K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005).
Champagne, D.L. et al. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 28, 6037–6045 (2008).
Weaver, I.C. et al. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854 (2004).
Chen, W.G. et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302, 885–889 (2003).
Martinowich, K. et al. DNA methylation–related chromatin remodeling in activity-dependent BDNF gene regulation. Science 302, 890–893 (2003).
Sadri-Vakili, G. et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J. Neurosci. 30, 11735–11744 (2010).
Chen-Plotkin, A.S. et al. Decreased association of the transcription factor Sp1 with genes downregulated in Huntington's disease. Neurobiol. Dis. 22, 233–241 (2006).
Braveman, M.W., Chen-Plotkin, A.S., Yohrling, G.J. & Cha, J.H. Chromatin immunoprecipitation technique for study of transcriptional dysregulation in intact mouse brain. Methods Mol. Biol. 277, 261–276 (2004).
Sadri-Vakili, G. et al. Histones associated with downregulated genes are hypo-acetylated in Huntington's disease models. Hum. Mol. Genet. 16, 1293–1306 (2007).
Acknowledgements
We thank R. Schassburger, T. Hopkins, B. Kimmey, S. Friedman, A. Lee, S. Darnell and G. Sangrey for technical assistance, and L. Briand for advice on experimental design. This work was supported by grants from the US National Institutes of Health (R01s DA15214, DA22339, DA33641, K02 DA18678, K01 DA30445, F31 DA31535, T32s DA28874 and MH86599).
Author information
Authors and Affiliations
Contributions
F.M.V., S.L.W., H.D.S. and G.S.-V. performed experiments. F.M.V. and R.C.P. analyzed the data, prepared the figures and wrote the first draft of the manuscript. All authors designed experiments and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 201 kb)
Rights and permissions
About this article
Cite this article
Vassoler, F., White, S., Schmidt, H. et al. Epigenetic inheritance of a cocaine-resistance phenotype. Nat Neurosci 16, 42–47 (2013). https://doi.org/10.1038/nn.3280
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3280
This article is cited by
-
Paternal cocaine-seeking motivation defines offspring’s vulnerability to addiction by down-regulating GABAergic GABRG3 in the ventral tegmental area
Translational Psychiatry (2024)
-
Peripheral neurotrophin levels during controlled crack/cocaine abstinence: a systematic review and meta-analysis
Scientific Reports (2024)
-
BDNF promotes mouse follicular development and reverses ovarian aging by promoting cell proliferation
Journal of Ovarian Research (2023)
-
Learning and memory deficits produced by aspartame are heritable via the paternal lineage
Scientific Reports (2023)
-
Engram neurons: Encoding, consolidation, retrieval, and forgetting of memory
Molecular Psychiatry (2023)