Key Points
-
Branched-chain amino acids (BCAAs) have beneficial nutrient signalling effects but paradoxically are associated with obesity, insulin resistance and type 2 diabetes mellitus (T2DM)
-
BCAAs might be a marker of, rather than, a cause of insulin resistance, as insulin resistance increases the rate of appearance of BCAAs and is linked to reduced expression of mitochondrial BCAA catabolic enzymes
-
Alternatively, two mechanisms have emerged indicating that a causative link exists between increased plasma concentrations of BCAAs and T2DM or insulin resistance
-
In the first mechanism, persistent activation of the mammalian target of rapamycin complex 1 signalling pathway uncouples the insulin receptor from the insulin signalling mediator, IRS-1, which leads to insulin resistance
-
In the second mechanism, abnormal BCAA metabolism in obesity results in accumulation of toxic BCAA metabolites that in turn trigger the mitochondrial dysfunction and stress signalling associated with insulin resistance and T2DM
-
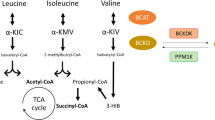
Factors that alter expression of genes involved in the BCAA metabolic pathway (or post-translational modification of the encoded proteins) are associated with obesity and T2DM; three genes in the pathway are candidate genes for obesity and/or T2DM
Abstract
Branched-chain amino acids (BCAAs) are important nutrient signals that have direct and indirect effects. Frequently, BCAAs have been reported to mediate antiobesity effects, especially in rodent models. However, circulating levels of BCAAs tend to be increased in individuals with obesity and are associated with worse metabolic health and future insulin resistance or type 2 diabetes mellitus (T2DM). A hypothesized mechanism linking increased levels of BCAAs and T2DM involves leucine-mediated activation of the mammalian target of rapamycin complex 1 (mTORC1), which results in uncoupling of insulin signalling at an early stage. A BCAA dysmetabolism model proposes that the accumulation of mitotoxic metabolites (and not BCAAs per se) promotes β-cell mitochondrial dysfunction, stress signalling and apoptosis associated with T2DM. Alternatively, insulin resistance might promote aminoacidaemia by increasing the protein degradation that insulin normally suppresses, and/or by eliciting an impairment of efficient BCAA oxidative metabolism in some tissues. Whether and how impaired BCAA metabolism might occur in obesity is discussed in this Review. Research on the role of individual and model-dependent differences in BCAA metabolism is needed, as several genes (BCKDHA, PPM1K, IVD and KLF15) have been designated as candidate genes for obesity and/or T2DM in humans, and distinct phenotypes of tissue-specific branched chain ketoacid dehydrogenase complex activity have been detected in animal models of obesity and T2DM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cota, D. et al. Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 (2006).
Blouet, C., Jo, Y. H., Li, X. & Schwartz, G. J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus–brainstem circuit. J. Neurosci. 29, 8302–8311 (2009).
Blouet, C. & Schwartz, G. J. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 16, 579–587 (2012).
Schwartz, G. J. Central leucine sensing in the control of energy homeostasis. Endocrinol. Metab. Clin. North Am. 42, 81–87 (2013).
Laeger, T. et al. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R310–R320 (2014).
Devkota, S. & Layman, D. K. Protein metabolic roles in treatment of obesity. Curr. Opin. Clin. Nutr. Metab. Care 13, 403–407 (2010).
Layman, D. K. & Walker, D. A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr. 136, 319S–323S (2006).
Norton, L. E. & Layman, D. K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 136, 533S–537S (2006).
Li, H., Xu, M., Lee, J., He, C. & Xie, Z. Leucine supplementation increases SIRT1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 303, E1234–E1244 (2012).
Li, Z. & Heber, D. Sarcopenic obesity in the elderly and strategies for weight management. Nutr. Rev. 70, 57–64 (2012).
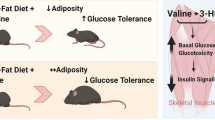
Guo, K., Yu, Y. H., Hou, J. & Zhang, Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr. Metab. (Lond.) 7, 57 (2010).
Zhang, Y. et al. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 56, 1647–1654 (2007).
Binder, E. et al. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity (Silver Spring) 22, 713–720 (2014).
Chen, H., Simar, D., Ting, J. H., Erkelens, J. R. & Morris, M. J. Leucine improves glucose and lipid status in offspring from obese dams, dependent on diet type, but not caloric intake. J. Neuroendocrinol. 24, 1356–1364 (2012).
Torres-Leal, F. L. et al. Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr. Metab. (Lond.) 8, 62 (2011).
Potier, M., Darcel, N. & Tome, D. Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 12, 54–58 (2009).
Lopez, N., Sanchez, J., Pico, C., Palou, A. & Serra, F. Dietary L-leucine supplementation of lactating rats results in a tendency to increase lean/fat ratio associated to lower orexigenic neuropeptide expression in hypothalamus. Peptides 31, 1361–1367 (2010).
Nairizi, A., She, P., Vary, T. C. & Lynch, C. J. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J. Nutr. 139, 715–719 (2009).
Stevanovic, D. et al. Intracerebroventricular administration of metformin inhibits ghrelin-induced hypothalamic AMP-kinase signalling and food intake. Neuroendocrinology 96, 24–31 (2012).
Chen, Q. & Reimer, R. A. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition 25, 340–349 (2009).
Macotela, Y. et al. Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 6, e21187 (2011).
Qin, L. Q. et al. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged East Asian and Western adults. J. Nutr. 141, 249–254 (2011).
Salehi, A. et al. The insulinogenic effect of whey protein is partially mediated by a direct effect of amino acids and GIP on β-cells. Nutr. Metab. (Lond.) 9, 48 (2012).
Xu, G. et al. Gastric mammalian target of rapamycin signaling regulates ghrelin production and food intake. Endocrinology 150, 3637–3644 (2009).
Lynch, C. J. et al. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 291, E621–E630 (2006).
Vary, T. C. & Lynch, C. J. Nutrient signaling components controlling protein synthesis in striated muscle. J. Nutr. 137, 1835–1843 (2007).
Dodd, K. M. & Tee, A. R. Leucine and mTORC1: a complex relationship. Am. J. Physiol. Endocrinol. Metab. 302, E1329–E1342 (2012).
Lynch, C. J. et al. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 283, E503–E513 (2002).
Lang, C. H., Frost, R. A., Bronson, S. K., Lynch, C. J. & Vary, T. C. Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. Am. J. Physiol. Endocrinol. Metab. 298, E1283–E1294 (2010).
Vary, T. C., Deiter, G. & Lynch, C. J. Rapamycin limits formation of active eukaryotic initiation factor 4F complex following meal feeding in rat hearts. J. Nutr. 137, 1857–1862 (2007).
Vary, T. C., Anthony, J. C., Jefferson, L. S., Kimball, S. R. & Lynch, C. J. Rapamycin blunts nutrient stimulation of eIF4G, but not PKCε phosphorylation, in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 293, E188–E196 (2007).
Vary, T. C. & Lynch, C. J. Meal feeding enhances formation of eIF4F in skeletal muscle: role of increased eIF4E availability and eIF4G phosphorylation. Am. J. Physiol. Endocrinol. Metab. 290, E631–E642 (2006).
Wang, X. & Proud, C. G. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21, 362–369 (2006).
Laplante, M. & Sabatini, D. M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 126, 1713–1719 (2013).
Garrow, J. S. The contribution of protein synthesis to thermogenesis in man. Int. J. Obes. 9 (Suppl. 2), 97–101 (1985).
Giordano, M. & Castellino, P. Correlation between amino acid induced changes in energy expenditure and protein metabolism in humans. Nutrition 13, 309–312 (1997).
Layman, D. K. & Baum, J. I. Dietary protein impact on glycemic control during weight loss. J. Nutr. 134, 968S–973S (2004).
Tsujinaka, T. et al. Modulation of thermogenic response to parenteral amino acid infusion in surgical stress. Nutrition 12, 36–39 (1996).
Yamaoka, I. Modification of core body temperature by amino acid administration. Asia Pac. J. Clin. Nutr. 17 (Suppl. 1), 309–311 (2008).
Pitkänen, O., Takala, J., Pöyhönen, M. & Kari, A. Branched-chain and mixed amino acid solutions and thermogenesis in postoperative patients. Nutrition 10, 132–137 (1994).
Kawaguchi, T., Taniguchi, E. & Sata, M. Effects of oral branched-chain amino acids on hepatic encephalopathy and outcome in patients with liver cirrhosis. Nutr. Clin. Pract. 28, 580–588 (2013).
Ichikawa, K. et al. Branched-chain amino acid-enriched nutrients stimulate antioxidant DNA repair in a rat model of liver injury induced by carbon tetrachloride. Mol. Biol. Rep. 39, 10803–10810 (2012).
Kuwahata, M. et al. Supplementation with branched-chain amino acids attenuates hepatic apoptosis in rats with chronic liver disease. Nutr. Res. 32, 522–529 (2012).
Hayaishi, S. et al. Oral branched-chain amino acid granules reduce the incidence of hepatocellular carcinoma and improve event-free survival in patients with liver cirrhosis. Dig. Dis. 29, 326–332 (2011).
Holecek, M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition 26, 482–490 (2010).
Layman, D. K. Dietary guidelines should reflect new understandings about adult protein needs. Nutr. Metab. (Lond.) 6, 12 (2009).
Mojtahedi, M. C. et al. The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J. Gerontol A Biol. Sci. Med. Sci. 66, 1218–1225 (2011).
Layman, D. K. et al. Defining meal requirements for protein to optimize metabolic roles of amino acids. Am. J. Clin. Nutr. http://dx.doi.org/10.3945/ajcn.114.084053.
Millward, D. J., Layman, D. K., Tome, D. & Schaafsma, G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am. J. Clin. Nutr. 87, 1576S–1581S (2008).
Muto, Y. et al. Overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol. Res. 35, 204–214 (2006).
Norton, L. E., Wilson, G. J., Layman, D. K., Moulton, C. J. & Garlick, P. J. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr. Metab. (Lond.) 9, 67 (2012).
Zeng, Y. J. et al. Characteristics and risk factors for hyperglycemia in Chinese female patients with systemic lupus erythematosus. Lupus 19, 1344–1350 (2010).
Wurtz, P. et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 61, 1372–1380 (2012).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Tai, E. S. et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53, 757–767 (2010).
Suhre, K. et al. Metabolic footprint of diabetes: a multiplatform metabolomics study in an epidemiological setting. PLoS ONE 5, e13953 (2010).
Schauder, P., Zavelberg, D., Langer, K. & Herbertz, L. Sex-specific differences in plasma branched-chain keto acid levels in obesity. Am. J. Clin. Nutr. 46, 58–60 (1987).
Pennetti, V., Galante, A., Zonta-Sgaramella, L. & Jayakar, S. D. Relation between obesity, insulinemia, and serum amino acid concentrations in a sample of Italian adults. Clin. Chem. 28, 2219–2224 (1982).
Newgard, C. B. et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326 (2009).
Newgard, C. B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 15, 606–614 (2012).
Nair, K. S., Garrow, J. S., Ford, C., Mahler, R. F. & Halliday, D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia 25, 400–403 (1983).
McCormack, S. E. et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 8, 52–61 (2013).
Lackey, D. E. et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am. J. Physiol. Endocrinol. Metab. 304, E1175–E1187 (2013).
Kim, M. J. et al. Obesity-related metabolomic analysis of human subjects in black soybean peptide intervention study by ultraperformance liquid chromatography and quadrupole-time-of-flight mass spectrometry. J. Obes. 2013, 874981 (2013).
Forlani, G. et al. Insulin-dependent metabolism of branched-chain amino acids in obesity. Metabolism 33, 147–150 (1984).
Fiehn, O. et al. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 5, e15234 (2010).
Felig, P., Marliss, E. & Cahill, G. F. Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 281, 811–816 (1969).
Cheng, S. et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 125, 2222–2231 (2012).
Caballero, B., Finer, N. & Wurtman, R. J. Plasma amino acids and insulin levels in obesity: response to carbohydrate intake and tryptophan supplements. Metabolism 37, 672–676 (1988).
Belfiore, F., Iannello, S. & Rabuazzo, A. M. Insulin resistance in obesity: a critical analysis at enzyme level. A review. Int. J. Obes. 3, 301–323 (1979).
Adibi, S. A. Influence of dietary deprivations on plasma concentration of free amino acids of man. J. Appl. Physiol. 25, 52–57 (1968).
She, P. et al. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 293, E1552–E1563 (2007).
She, P. et al. Leucine and protein metabolism in obese Zucker rats. PLoS ONE 8, e59443 (2013).
Herman, M. A., She, P., Peroni, O. D., Lynch, C. J. & Kahn, B. B. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 285, 11348–11356 (2010).
Olson, K. C., Chen, G., Xu, Y., Hajnal, A. & Lynch, C. J. Alloisoleucine differentiates the branched-chain aminoacidemia of Zucker and dietary obese rats. Obesity (Silver Spring) 22, 1212–1215 (2014).
Batch, B. C. et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 62, 961–969 (2013).
Huang, Y., Zhou, M., Sun, H. & Wang, Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovasc. Res. 90, 220–223 (2011).
Breitman, I. et al. The effects of an amino acid supplement on glucose homeostasis, inflammatory markers, and incretins after laparoscopic gastric bypass. J. Am. Coll. Surg. 212, 617–625 (2011).
Badoud, F. et al. Serum and adipose tissue amino Acid homeostasis in the metabolically healthy obese. J. Proteome Res. 13, 3455–3466 (2014).
Fernstrom, J. D. Branched-chain amino acids and brain function. J. Nutr. 135, 1539S–1546S (2005).
Mans, A. M., DeJoseph, M. R., Davis, D. W. & Hawkins, R. A. Regional amino acid transport into brain during diabetes: effect of plasma amino acids. Am. J. Physiol. 253, E575–E583 (1987).
Crandall, E. A. & Fernstrom, J. D. Effect of experimental diabetes on the levels of aromatic and branched-chain amino acids in rat blood and brain. Diabetes 32, 222–230 (1983).
Fernstrom, M. H., Volk, E. A. & Fernstrom, J. D. In vivo inhibition of tyrosine uptake into rat retina by large neutral but not acidic amino acids. Am. J. Physiol. 251, E393–E399 (1986).
Coppola, A. et al. Branched-chain amino acids alter neurobehavioral function in rats. Am. J. Physiol. Endocrinol. Metab. 304, E405–E413 (2013).
Pan, A. et al. Bidirectional association between depression and obesity in middle-aged and older women. Int. J. Obes. (Lond.) 36, 595–602 (2012).
Blommaart, E. F., Luiken, J. J., Blommaart, P. J., van Woerkom, G. M. & Meijer, A. J. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J. Biol. Chem. 270, 2320–2326 (1995).
Maki, T. et al. Branched-chain amino acids reduce hindlimb suspension-induced muscle atrophy and protein levels of atrogin-1 and MuRF1 in rats. Nutr. Res. 32, 676–683 (2012).
May, M. E. & Buse, M. G. Effects of branched-chain amino acids on protein turnover. Diabetes Metab. Rev. 5, 227–245 (1989).
Mordier, S., Deval, C., Bechet, D., Tassa, A. & Ferrara, M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J. Biol. Chem. 275, 29900–29906 (2000).
Mortimore, G. E. & Poso, A. R. Lysosomal pathways in hepatic protein degradation: regulatory role of amino acids. Fed. Proc. 43, 1289–1294 (1984).
Sugawara, T., Ito, Y., Nishizawa, N. & Nagasawa, T. Regulation of muscle protein degradation, not synthesis, by dietary leucine in rats fed a protein-deficient diet. Amino Acids 37, 609–616 (2009).
Hong, S. O. & Layman, D. K. Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles. J. Nutr. 114, 1204–1212 (1984).
Adegoke, O. A. et al. Fed-state clamp stimulates cellular mechanisms of muscle protein anabolism and modulates glucose disposal in normal men. Am. J. Physiol. Endocrinol. Metab. 296, E105–E113 (2009).
Baptista, I. L. et al. Leucine attenuates skeletal muscle wasting via inhibition of ubiquitin ligases. Muscle Nerve 41, 800–808 (2010).
Glass, D. J. Signaling pathways perturbing muscle mass. Curr. Opin. Clin. Nutr. Metab. Care 13, 225–229 (2010).
Latres, E. et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J. Biol. Chem. 280, 2737–2744 (2005).
Paula-Gomes, S. et al. Insulin suppresses atrophy and autophagy-related genes in heart tissue and cardiomyocytes through AKT/FOXO signaling. Horm. Metab. Res. 45, 849–855 (2013).
Williamson, J. R., Walajtys-Rode, E. & Coll., K. E. Effects of branched chain α-ketoacids on the metabolism of isolated rat liver cells. I. Regulation of branched chain α-ketoacid metabolism. J. Biol. Chem. 254, 11511–11520 (1979).
Greenhaff, P. L. et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am. J. Physiol. Endocrinol. Metab. 295, E595–E604 (2008).
O'Connor, P. M., Bush, J. A., Suryawan, A., Nguyen, H. V. & Davis, T. A. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 284, E110–E119 (2003).
Fryburg, D. A., Jahn, L. A., Hill, S. A., Oliveras, D. M. & Barrett, E. J. Insulin and insulin-like growth factor-I enhance human skeletal muscle protein anabolism during hyperaminoacidemia by different mechanisms. J. Clin. Invest. 96, 1722–1729 (1995).
Estornell, E., Cabo, J. & Barber, T. Protein synthesis is stimulated in nutritionally obese rats. J. Nutr. 125, 1309–1315 (1995).
Guillet, C., Masgrau, A. & Boirie, Y. Is protein metabolism changed with obesity? Curr. Opin. Clin. Nutr. Metab. Care 14, 89–92 (2011).
Kumashiro, N. et al. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62, 2183–2194 (2013).
Nilsson, M. I. et al. Abnormal protein turnover and anabolic resistance to exercise in sarcopenic obesity. FASEB J. 27, 3905–3916 (2013).
Welle, S., Barnard, R. R., Statt, M. & Amatruda, J. M. Increased protein turnover in obese women. Metabolism 41, 1028–1034 (1992).
Louard, R. J., Barrett, E. J. & Gelfand, R. A. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 44, 424–429 (1995).
Hung, C. F. et al. Relationship between obesity and the risk of clinically significant depression: Mendelian randomisation study. Br. J. Psychiatry 205, 24–28 (2014).
Um, S. H. et al. Absence of S6K1 protects against age and diet-induced obesity while enhancing insulin sensitivity. Nature 431, 200–205 (2004).
Um, S. H., D'Alessio, D. & Thomas, G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab. 3, 393–402 (2006).
She, P. et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 6, 181–194 (2007).
Kihlberg, R., Bark, S. & Hallberg, D. An oral amino acid loading test before and after intestinal bypass operation for morbid obesity. Acta Chir. Scand. 148, 73–86 (1982).
Magkos, F. et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 62, 2757–2761 (2013).
Laferrere, B. et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 3, 80re2 (2011).
Doi, M. et al. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J. Nutr. 135, 2103–2108 (2005).
Doi, M., Yamaoka, I., Nakayama, M., Sugahara, K. & Yoshizawa, F. Hypoglycemic effect of isoleucine involves increased muscle glucose uptake and whole body glucose oxidation and decreased hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 292, E1683–E1693 (2007).
Broca, C. et al. Insulinotropic agent ID-1101 (4-hydroxyisoleucine) activates insulin signaling in rat. Am. J. Physiol. Endocrinol. Metab. 287, E463–E471 (2004).
Blanchard, P. G. et al. Major involvement of mTOR in the PPARγ-induced stimulation of adipose tissue lipid uptake and fat accretion. J. Lipid Res. 53, 1117–1125 (2012).
Herder, C. & Roden, M. Genetics of type 2 diabetes: pathophysiologic and clinical relevance. Eur. J. Clin. Invest. 41, 679–692 (2011).
Tiffin, N. et al. Computational disease gene identification: a concert of methods prioritizes type 2 diabetes and obesity candidate genes. Nucleic Acids Res. 34, 3067–3081 (2006).
Vimaleswaran, K. S. et al. Candidate genes for obesity-susceptibility show enriched association within a large genome-wide association study for BMI. Hum. Mol. Genet. 21, 4537–4542 (2012).
Leibowitz, G., Cerasi, E. & Ketzinel-Gilad, M. The role of mTOR in the adaptation and failure of β-cells in type 2 diabetes. Diabetes Obes. Metab. 10 (Suppl. 4), 157–169 (2008).
Lopes, P. et al. Effects of cyclosporine and sirolimus on insulin-stimulated glucose transport and glucose tolerance in a rat model. Transplant Proc. 45, 1142–1148 (2013).
Dandel, M., Lehmkuhl, H. B., Knosalla, C. & Hetzer, R. Impact of different long-term maintenance immunosuppressive therapy strategies on patients' outcome after heart transplantation. Transpl. Immunol. 23, 93–103 (2010).
Gyurus, E., Kaposztas, Z. & Kahan, B. D. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: a long-term analysis of various treatment regimens. Transplant Proc. 43, 1583–1592 (2011).
Hughes, K. J. & Kennedy, B. K. Rapamycin paradox resolved. Science 335, 1578–1579 (2012).
Lamming, D. W. et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335, 1638–1643 (2012).
Sarbassov, D. D. et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 (2006).
Pham, P.-T. T., Pham, P.-M. T., Pham, S. V., Pham, P.-A. T. & Pham, P.-C. T. New onset diabetes after transplantation (NODAT): an overview. Diabetes Metab. Syndr. Obes. 4, 175–186 (2011).
Bridi, R. et al. α-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab. Brain Dis. 20, 155–167 (2005).
Funchal, C. et al. Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain α-keto acids accumulating in maple syrup urine disease. Neurochem. Int. 49, 640–650 (2006).
Jackson, R. H. & Singer, T. P. Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids. J. Biol. Chem. 258, 1857–1865 (1983).
Walajtys-Rode, E. & Williamson, J. R. Effects of branched chain α-ketoacids on the metabolism of isolated rat liver cells. III. Interactions with pyruvate dehydrogenase. J. Biol. Chem. 255, 413–418 (1980).
Jouvet, P., Kozma, M. & Mehmet, H. Primary human fibroblasts from a maple syrup urine disease patient undergo apoptosis following exposure to physiological concentrations of branched chain amino acids. Ann. NY Acad. Sci. 926, 116–121 (2000).
Jouvet, P. et al. Maple syrup urine disease metabolites induce apoptosis in neural cells without cytochrome c release or changes in mitochondrial membrane potential. Biochem. Soc. Trans. 26, S341 (1998).
Kasinski, A., Doering, C. B. & Danner, D. J. Leucine toxicity in a neuronal cell model with inhibited branched chain amino acid catabolism. Brain Res. Mol. Brain Res. 122, 180–187 (2004).
Lu, G. et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J. Clin. Invest. 119, 1678–1687 (2009).
Oyarzabal, A. et al. A novel regulatory defect in the branched-chain α-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum. Mutat. 34, 355–362 (2013).
Lu, G. et al. Functional characterization of a mitochondrial Ser/Thr protein phosphatase in cell death regulation. Methods Enzymol. 457, 255–273 (2009).
Lu, G. et al. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 21, 784–796 (2007).
Amaral, A. U. et al. α-ketoisocaproic acid and leucine provoke mitochondrial bioenergetic dysfunction in rat brain. Brain Res. 1324, 75–84 (2010).
Adams, S. H. et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 139, 1073–1081 (2009).
Adams, S. H. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv. Nutr. 2, 445–456 (2011).
Shah, S. H. et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia 55, 321–330 (2012).
Wajner, M. & Goodman, S. I. Disruption of mitochondrial homeostasis in organic acidurias: insights from human and animal studies. J. Bioenerg. Biomembr. 43, 31–38 (2011).
Klimcakova, E. et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. J. Clin. Endocrinol. Metab. 96, E73–E82 (2011).
Pietilainen, K. H. et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med. 5, e51 (2008).
Leskinen, T. et al. Differences in muscle and adipose tissue gene expression and cardio-metabolic risk factors in the members of physical activity discordant twin pairs. PLoS ONE 5, e12609 (2010).
Lan, H. et al. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes 52, 688–700 (2003).
Stancakova, A. et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 61, 1895–1902 (2012).
Zimmerman, H. A., Olson, K. C., Chen, G. & Lynch, C. J. Adipose transplant for inborn errors of branched chain amino acid metabolism in mice. Mol. Genet. Metab. 109, 345–353 (2013).
Mazariegos, G. V. et al. Liver transplantation for classical maple syrup urine disease: long-term follow-up in 37 patients and comparative United Network for Organ Sharing experience. J. Pediatr. 160, 116–121 (2012).
Harris, R. A., Joshi, M., Jeoung, N. H. & Obayashi, M. Overview of the molecular and biochemical basis of branched-chain amino acid catabolism. J. Nutr. 135, 1527S–1530S (2005).
Lefort, N. et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle. Diabetes 59, 2444–2452 (2010).
Mullen, E. & Ohlendieck, K. Proteomic profiling of non-obese type 2 diabetic skeletal muscle. Int. J. Mol. Med. 25, 445–458 (2010).
Roe, C. R. et al. Isolated isobutyryl-CoA dehydrogenase deficiency: an unrecognized defect in human valine metabolism. Mol. Genet. Metab. 65, 264–271 (1998).
Shin, A. C. et al. Brain insulin lowers circulating BCAA levels by inducing hepatic branched-chain α keto-acid dehydrogenase. Cell Metab. (in press).
Tschop, M. H. et al. A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 (2012).
Wolfe, R. R. & Chinkes, D. L. in Isotope Tracers in Metabolic Research 299–323 (Wiley, 2005).
Allsop, J. R., Wolfe, R. R. & Burke, J. F. Tracer priming the bicarbonate pool. J. Appl. Physiol. 45, 137–139 (1978).
Doisaki, M. et al. Regulation of hepatic branched-chain α-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Biochem. Biophys. Res. Commun. 393, 303–307 (2010).
Kuzuya, T. et al. Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Biochem. Biophys. Res. Commun. 373, 94–98 (2008).
Kadota, Y., Toyoda, T., Kitaura, Y., Adams, S. H. & Shimomura, Y. Regulation of hepatic branched-chain α-ketoacid dehydrogenase complex in rats fed a high-fat diet. Obes. Res. Clin. Pract. 7, e439–e444 (2013).
Joshi, M., Jeoung, N. H., Popov, K. M. & Harris, R. A. Identification of a novel PP2C-type mitochondrial phosphatase. Biochem. Biophys. Res. Commun. 356, 38–44 (2007).
Taneera, J. et al. A systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metab. 16, 122–134 (2012).
Kettunen, J. et al. Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat. Genet. 44, 269–276 (2012).
Xu, M. et al. Genetic determinant for amino acid metabolites and changes in body weight and insulin resistance in response to weight-loss diets: the preventing overweight using novel dietary strategies (POUNDS LOST) trial. Circulation 127, 1283–1289 (2013).
Cipolla-Neto, J., Amaral, F. G., Afeche, S. C., Tan, D. X. & Reiter, R. J. Melatonin, energy metabolism, and obesity: a review. J. Pineal Res. 56, 371–381 (2014).
Shi, S. Q., Ansari, T. S., McGuinness, O. P., Wasserman, D. H. & Johnson, C. H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 23, 372–381 (2013).
Wu, L. & Reddy, A. B. Disrupting rhythms: diet-induced obesity impairs diurnal rhythms in metabolic tissues. Diabetes 62, 1829–1830 (2013).
Holt, R. I., Barnett, A. H. & Bailey, C. J. Bromocriptine: old drug, new formulation and new indication. Diabetes Obes. Metab. 12, 1048–1057 (2010).
Cano, P. et al. Effect of a high-fat diet on 24-hour pattern of circulating adipocytokines in rats. Obesity (Silver Spring) 17, 1866–1871 (2009).
Takashima, M. et al. Role of KLF15 in regulation of hepatic gluconeogenesis and metformin action. Diabetes 59, 1608–1615 (2010).
Elbein, S. C. et al. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes 60, 1019–1029 (2011).
Corkey, B. E., Martin-Requero, A., Walajtys-Rode, E., Williams, R. J. & Williamson, J. R. Regulation of the branched chain α-ketoacid pathway in liver. J. Biol. Chem. 257, 9668–9676 (1982).
Hu, H., Jaskiewicz, J. A. & Harris, R. A. Ethanol and oleate inhibition of α-ketoisovalerate and 3-hydroxyisobutyrate metabolism by isolated hepatocytes. Arch. Biochem. Biophys. 299, 57–62 (1992).
Koves, T. R. et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56 (2008).
Frohnert, B. I. & Bernlohr, D. A. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Adv. Nutr. 4, 157–163 (2013).
Frohnert, B. I. et al. Increased adipose protein carbonylation in human obesity. Obesity (Silver Spring) 19, 1735–1741 (2011).
Long, E. K., Olson, D. M. & Bernlohr, D. A. High-fat diet induces changes in adipose tissue trans-4-oxo-2-nonenal and trans-4-hydroxy-2-nonenal levels in a depot-specific manner. Free Radic. Biol. Med. 63, 390–398 (2013).
Ruskovska, T. & Bernlohr, D. A. Oxidative stress and protein carbonylation in adipose tissue—implications for insulin resistance and diabetes mellitus. J. Proteomics 92, 323–334 (2013).
Mamer, O. A. & Reimer, M. L. On the mechanisms of the formation of L-alloisoleucine and the 2-hydroxy-3-methylvaleric acid stereoisomers from L-isoleucine in maple syrup urine disease patients and in normal humans. J. Biol. Chem. 267, 22141–22147 (1992).
Zhang, B., Zhao, Y., Harris, R. A. & Crabb, D. W. Molecular defects in the E1 α subunit of the branched-chain α-ketoacid dehydrogenase complex that cause maple syrup urine disease. Mol. Biol. Med. 8, 39–47 (1991).
Strauss, K. A. et al. Maple syrup urine disease. GeneReviews [online], (1993).
Strauss, K. A. et al. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol. Genet. Metab. 99, 333–345 (2010).
Homanics, G. E., Skvorak, K., Ferguson, C., Watkins, S. & Paul, H. S. Production and characterization of murine models of classic and intermediate maple syrup urine disease. BMC Med. Genet. 7, 33 (2006).
Zinnanti, W. J. et al. Dual mechanism of brain injury and novel treatment strategy in maple syrup urine disease. Brain 132, 903–918 (2009).
Bridi, R. et al. Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int. J. Dev. Neurosci. 21, 327–332 (2003).
Kobayashi, R. et al. Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Arch. Biochem. Biophys. 407, 231–240 (2002).
Shimomura, Y. et al. Branched-chain 2-oxo acid dehydrogenase complex activation by tetanic contractions in rat skeletal muscle. Biochim. Biophys. Acta 1157, 290–296 (1993).
Shimomura, Y. et al. Branched-chain amino acid catabolism in exercise and liver disease. J. Nutr. 136, 250S–253S (2006).
Wang, X. & Price, S. R. Differential regulation of branched-chain α-ketoacid dehydrogenase kinase expression by glucocorticoids and acidification in LLC-PK1-GR101 cells. Am. J. Physiol. Renal Physiol. 286, F504–F508 (2004).
Block, K. P., Richmond, W. B., Mehard, W. B. & Buse, M. G. Glucocorticoid-mediated activation of muscle branched-chain α-keto acid dehydrogenase in vivo. Am. J. Physiol. 252, E396–E407 (1987).
Popov, K. M. et al. Dietary control and tissue specific expression of branched-chain α-ketoacid dehydrogenase kinase. Arch. Biochem. Biophys. 316, 148–154 (1995).
Kobayashi, R. et al. Hepatic branched-chain α-keto acid dehydrogenase complex in female rats: activation by exercise and starvation. J. Nutr. Sci. Vitaminol. (Tokyo) 45, 303–309 (1999).
Zhao, Y. et al. Effect of dietary protein on the liver content and subunit composition of the branched-chain α-ketoacid dehydrogenase complex. Arch. Biochem. Biophys. 308, 446–453 (1994).
Nellis, M. M., Doering, C. B., Kasinski, A. & Danner, D. J. Insulin increases branched-chain α-ketoacid dehydrogenase kinase expression in Clone 9 rat cells. Am. J. Physiol. Endocrinol. Metab. 283, E853–E860 (2002).
Harris, R. A. et al. Regulation of the branched-chain α-ketoacid dehydrogenase and elucidation of a molecular basis for maple syrup urine disease. Adv. Enzyme Regul. 30, 245–263 (1990).
Harper, A. E., Miller, R. H. & Block, K. P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 4, 409–454 (1984).
Paxton, R. & Harris, R. A. Regulation of branched-chain α-ketoacid dehydrogenase kinase. Arch. Biochem. Biophys. 231, 48–57 (1984).
Nawabi, M. D., Block, K. P., Chakrabarti, M. C. & Buse, M. G. Administration of endotoxin, tumor necrosis factor, or interleukin 1 to rats activates skeletal muscle branched-chain α-keto acid dehydrogenase. J. Clin. Invest. 85, 256–263 (1990).
Harris, R. A., Kobayashi, R., Murakami, T. & Shimomura, Y. Regulation of branched-chain α-keto acid dehydrogenase kinase expression in rat liver. J. Nutr. 131, 841S–845S (2001).
Shimomura, Y., Obayashi, M., Murakami, T. & Harris, R. A. Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain α-keto acid dehydrogenase kinase. Curr. Opin. Clin. Nutr. Metab. Care 4, 419–423 (2001).
Harris, R. A. et al. in Lipoic Acid: Energy Production, Antioxidant Activity and Health Effects (eds Patel, M. S. & Packer, L.) 101–148 (CRC Press, 2008).
Gillim, S. E., Paxton, R., Cook, G. A. & Harris, R. A. Activity state of the branched chain α-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats. Biochem. Biophys. Res. Commun. 111, 74–81 (1983).
Lombardo, Y. B., Serdikoff, C., Thamotharan, M., Paul, H. S. & Adibi, S. A. Inverse alterations of BCKA dehydrogenase activity in cardiac and skeletal muscles of diabetic rats. Am. J. Physiol. 277, E685–E692 (1999).
Lombardo, Y. B., Thamotharan, M., Bawani, S. Z., Paul, H. S. & Adibi, S. A. Posttranscriptional alterations in protein masses of hepatic branched-chain keto acid dehydrogenase and its associated kinase in diabetes. Proc. Assoc. Am. Physicians 110, 40–49 (1998).
Kobayashi, R., Shimomura, Y., Otsuka, M., Popov, K. M. & Harris, R. A. Experimental hyperthyroidism causes inactivation of the branched-chain α-ketoacid dehydrogenase complex in rat liver. Arch. Biochem. Biophys. 375, 55–61 (2000).
Harris, R. A., Powell, S. M., Paxton, R., Gillim, S. E. & Nagae, H. Physiological covalent regulation of rat liver branched-chain α-ketoacid dehydrogenase. Arch. Biochem. Biophys. 243, 542–555 (1985).
Hsiao, G. et al. Multi-tissue, selective PPARγ modulation of insulin sensitivity and metabolic pathways in obese rats. Am. J. Physiol. Endocrinol. Metab. 300, E164–E174 (2011).
Acknowledgements
C.J.L. acknowledges research support from the NIH (DK091784 and DK084428). S.H.A. acknowledges research support from the USDA–Agricultural Research Service (Intramural Project 5,306-51530-019-00) and the NIH (NIH-NIDDK R01DK078328). The authors wish to thank their many colleagues, collaborators and mentors who have inspired their interest in branched-chain amino acids and metabolic disease.
Author information
Authors and Affiliations
Contributions
C.J.L. and S.H.A. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
C.J.L. has received an honorarium for being a panelist for the Protein Summit, Washington, DC, USA, in 2013. S.H.A. declares no competing interests.
Rights and permissions
About this article
Cite this article
Lynch, C., Adams, S. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10, 723–736 (2014). https://doi.org/10.1038/nrendo.2014.171
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.171
This article is cited by
-
Abomasal infusion of branched-chain amino acids or branched-chain keto-acids alter lactation performance and liver triglycerides in fresh cows
Journal of Animal Science and Biotechnology (2024)
-
The Role of Branched-chain Amino Acids and Their Metabolism in Cardiovascular Diseases
Journal of Cardiovascular Translational Research (2024)
-
Amino acid profiles: exploring their diagnostic and pathophysiological significance in hypertension
Molecular Biology Reports (2024)
-
Serum metabolomics profiling by proton nuclear magnetic resonance spectroscopy reveals sexual dimorphism and masculinization of intermediate metabolism in women with polycystic ovary syndrome (PCOS)
Biology of Sex Differences (2023)
-
Dietary branched-chain amino acids intake, glycemic markers, metabolic profile, and anthropometric features in a community-based sample of overweight and obese adults
BMC Endocrine Disorders (2023)